Question: Only 1000% sure experts solve it correctly completes solutions not chat gpt okkk handwriting okk Remember: Moral Ascendancy Place it along with your signature in
Only 1000% sure experts solve it correctly completes solutions not chat gpt okkk handwriting okk
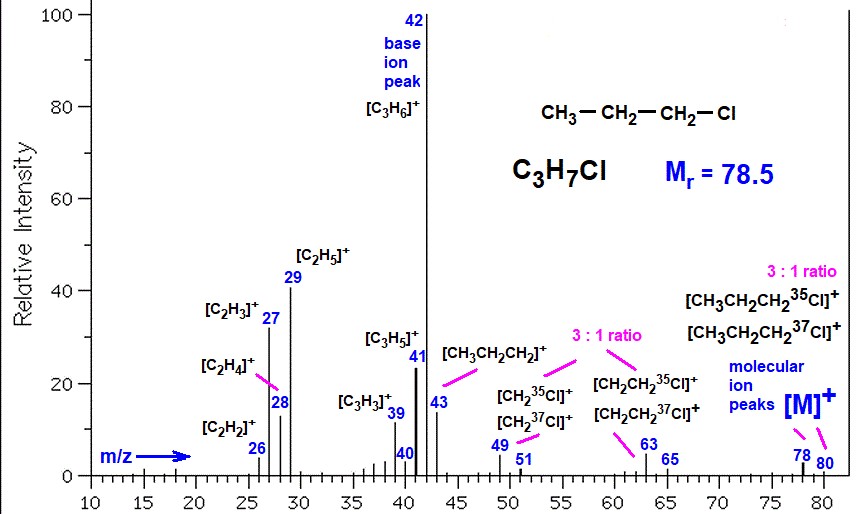
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


