Question: *Only Answer parts a,b, and d using MATLAB only Pure butanol is to be fed into a semibatch reactor containing pure ethyl acetate to produce
*Only Answer parts a,b, and d using MATLAB only
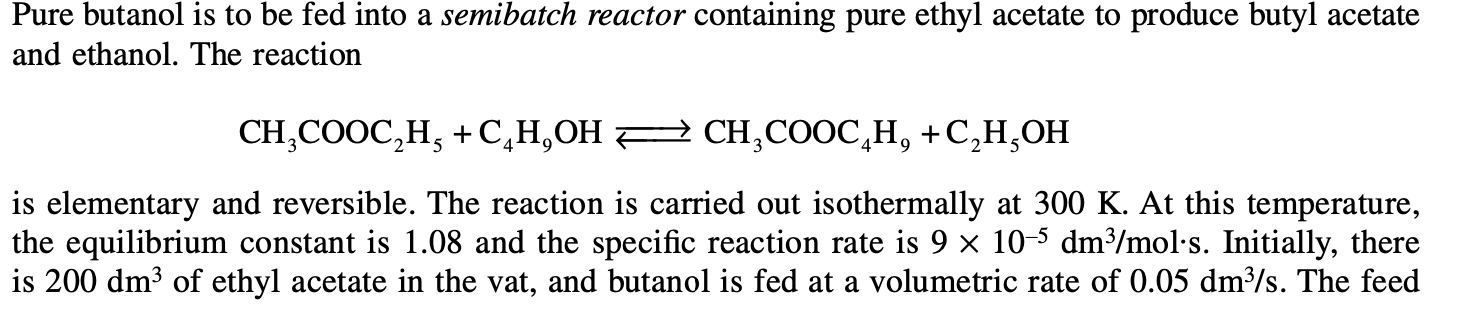

Pure butanol is to be fed into a semibatch reactor containing pure ethyl acetate to produce butyl acetate and ethanol. The reaction CH3COOC2H5+C4H9OHCH3COOC4H9+C2H5OH is elementary and reversible. The reaction is carried out isothermally at 300K. At this temperature, the equilibrium constant is 1.08 and the specific reaction rate is 9105dm3/mols. Initially, there is 200dm3 of ethyl acetate in the vat, and butanol is fed at a volumetric rate of 0.05dm3/s. The feed (a) Plot and analyze the equilibrium conversion of ethyl acetate as a function of time. (b) Plot and analyze the conversion of ethyl acetate, the rate of reaction, and the concentration of butanol as a function of time. (c) Rework part (b), assuming that ethanol evaporates (reactive distillation) as soon as it forms. (This is a graduate level question.) (d) Use Polymath or some other ODE solver to learn the sensitivity of conversion to various combinations of parameters (e.g., vary FB0:vA0,v0 ). (e) Apply one or more of the six ideas in Preface Table P-4, page xxviii, to this problem. (f) Write a question that requires critical thinking and then explain why your question requires critical thinking. (Hint: See Preface Section I.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


