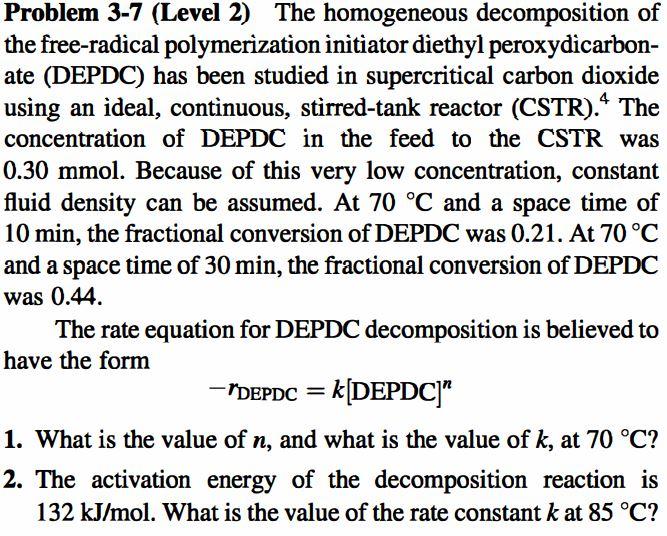
Question: Only B part please Problem 3-7 (Level 2) The homogeneous decomposition of the free-radical polymerization initiator diethyl peroxydcarbon- ate (DEPDC) has been studied in supercritical
Only B part please
Problem 3-7 (Level 2) The homogeneous decomposition of the free-radical polymerization initiator diethyl peroxydcarbon- ate (DEPDC) has been studied in supercritical carbon dioxide using an ideal, continuous, stirred-tank reactor (CSTR). The concentration of DEPDC in the feed to the CSTR was 0.30 mmol. Because of this very low concentration, constant fluid density can be assumed. At 70 C and a space time of 10 min, the fractional conversion of DEPDC was 0.21. At 70 C and a space time of 30 min, the fractional conversion of DEPDC was 0.44. The rate equation for DEPDC decomposition is believed to have the form - "DEPDC = k[DEPDC)" 1. What is the value of n, and what is the value of k, at 70 C? 2. The activation energy of the decomposition reaction is 132 kJ/mol. What is the value of the rate constant k at 85 C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


