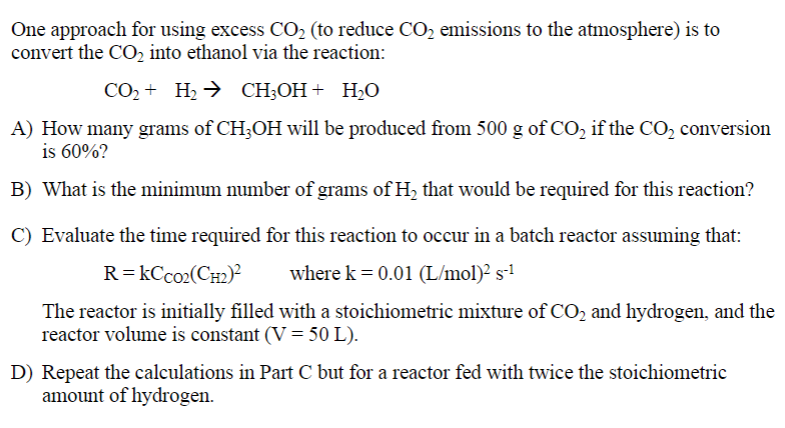
Question: only C and D please One approach for using excess CO2 (to reduce CO2 emissions to the atmosphere) is to convert the CO2 into ethanol
 only C and D please
only C and D please
One approach for using excess CO2 (to reduce CO2 emissions to the atmosphere) is to convert the CO2 into ethanol via the reaction: CO2+H2CH3OH+H2O A) How many grams of CH3OH will be produced from 500g of CO2 if the CO2 conversion is 60% ? B) What is the minimum number of grams of H2 that would be required for this reaction? C) Evaluate the time required for this reaction to occur in a batch reactor assuming that: R=kCCCO2(CH2)2wherek=0.01(L/mol)2s1 The reactor is initially filled with a stoichiometric mixture of CO2 and hydrogen, and the reactor volume is constant (V=50L). D) Repeat the calculations in Part C but for a reactor fed with twice the stoichiometric amount of hydrogen
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


