Question: ONLY NEED QUESTIONS 3 and 4 Thank you! Data Table: 419.89 Volume of H2O in your calorimeter Mass of H2O in your calorimeter Initial temp
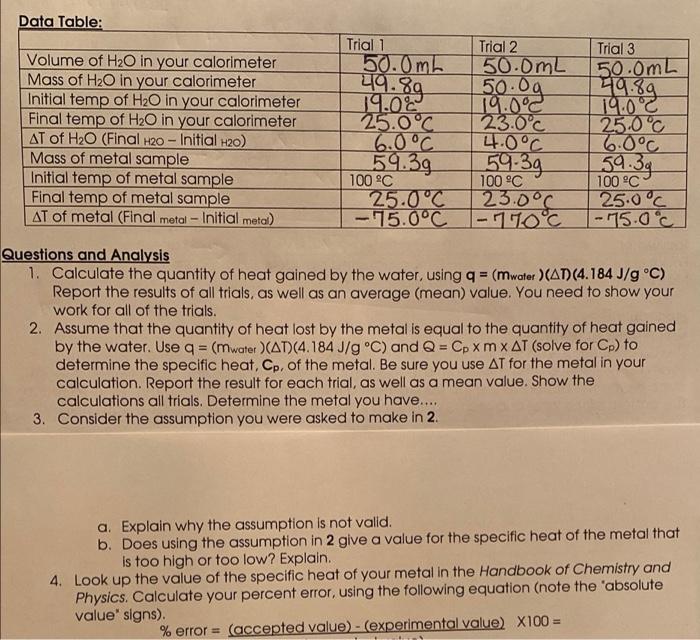
Data Table: 419.89 Volume of H2O in your calorimeter Mass of H2O in your calorimeter Initial temp of H2O in your calorimeter Final temp of H2O in your calorimeter AT of H2O (Final H20 - Initial H2O) Mass of metal sample Initial temp of metal sample Final temp of metal sample AT of metal (Final metal - Initial metal) Trial 1 Trial 2 50.0 mb 50.0mL 50.00 19.02 19.0C 25.0C 23.0C 6.0C 4.0C 59.39 100 C 100 C 25.0C 23.0C - 15.0C -110C Trial 3 50.0mL 49.89 19:00 25.0C 6.0C 59.39 100 C 25.0C |-75.0c 59.39 - Questions and Analysis 1. Calculate the quantity of heat gained by the water, using q = (mwater )(AD) (4.184 J/g C) Report the results of all trials, as well as an average (mean) value. You need to show your work for all of the trials. 2. Assume that the quantity of heat lost by the metal is equal to the quantity of heat gained by the water. Use q = (mwater (AT)(4.184J/g C) and Q = Cp XM X AT (solve for Cp) to determine the specific heat, Cp, of the metal. Be sure you use AT for the metal in your calculation. Report the result for each trial, as well as a mean value. Show the calculations all trials. Determine the metal you have..... 3. Consider the assumption you were asked to make in 2. a. Explain why the assumption is not valid. b. Does using the assumption in 2 give a value for the specific heat of the metal that is too high or too low? Explain. 4. Look up the value of the specific heat of your metal in the Handbook of Chemistry and Physics. Calculate your percent error, using the following equation (note the 'absolute value' signs). % error = (accepted value) - (experimental value) X100=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


