Question: ONLY PART A). not part b answer: i need help aolving the problem and getting to the answer (only part a) 0. The effluent from

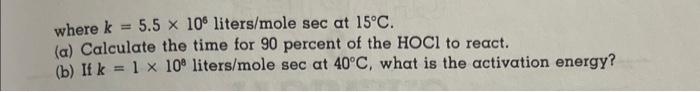
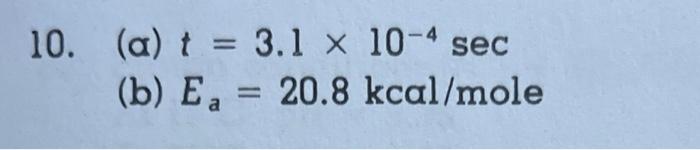
0. The effluent from a secondary clarifier has a pH of 8.3, an ammonia (NH3) concentration of 34mg liter, and is dosed with 103MHOCl. The reaction is reversible but assume conditions are such that only the forward reaction must be considered. NH3+HOClNH2Cl+H2O It has a rate law, dtd[NH3]=k[NH3][HOCl] where k=5.5106 liters /molesec at 15C. (a) Calculate the time for 90 percent of the HOCl to react. (b) If k=110 liters/mole sec at 40C, what is the activation energy? (a) t=3.1104sec (b) Ea=20.8kcal/mole
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


