Question: ONLY USE C LANGUAGE (NO OTHER LANGUAGE PYTHON C++ ETC) The pressure of a gas changes as the volume and temperature of the gas vary.
ONLY USE C LANGUAGE (NO OTHER LANGUAGE PYTHON C++ ETC)
The pressure of a gas changes as the volume and temperature of the gas vary.
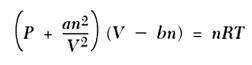
Write a program that uses the Van der Waals equation of state for a gas given above, to produce output that displays in tabular form the relationship between the pressure and the volume of n moles of carbon dioxide at a constant absolute temperature (T). Where P is the pressure in atmospheres, and V is the volume in litres. The Van der Waals constants for carbon dioxide are a = 3.592 L2 atm/mol2 and b = 0.0427 L/mol.
Use 0.08206 L atm/mol. K for the gas constant R.
Inputs to the program include n, the Kelvin temperature T, the initial and final volumes in millilitres, and the volume increment between lines of the table.
Your program will output a table in an output file (lets say output.txt) that varies the volume of the gas from the initial to the final volume, and the Pressure in steps prescribed by the volume increment.
Here is a sample run:
A sample run with input and output is given below:
Please enter at the prompts the number of moles of carbon dioxide > 0.02
a)the absolute temperature (kelvin) > 300
b)the initial volume in millilitres > 400
c)the final volume > 600
d)the increment volume between lines of the table > 50
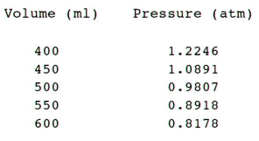
Content of the output file:
0.0200 moles of carbon dioxide at 300 kelvin
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


