Question: O=NO: Create the Lewis structure of NO2 - using the following steps. NOTE: do only the step asked for in each part- don't work ahead
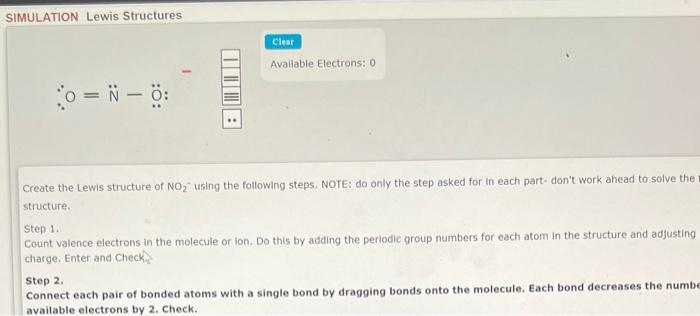
O=NO: Create the Lewis structure of NO2 - using the following steps. NOTE: do only the step asked for in each part- don't work ahead to solve the structure. Step 1. Count valence electrons in the molecule or lon. Do this by adding the periodic group numbers for each atom in the structure and adjusting charge. Enter and Check step 2. Connect each pair of bonded atoms with a single bond by dragging bonds onto the molecule. Each bond decreases the numb available electrons by 2 . Check
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


