Question: Open Answer questions 12 18 ( 2 points each ) are based on the data given below: The 2 H NMR spectrum shown right (s=
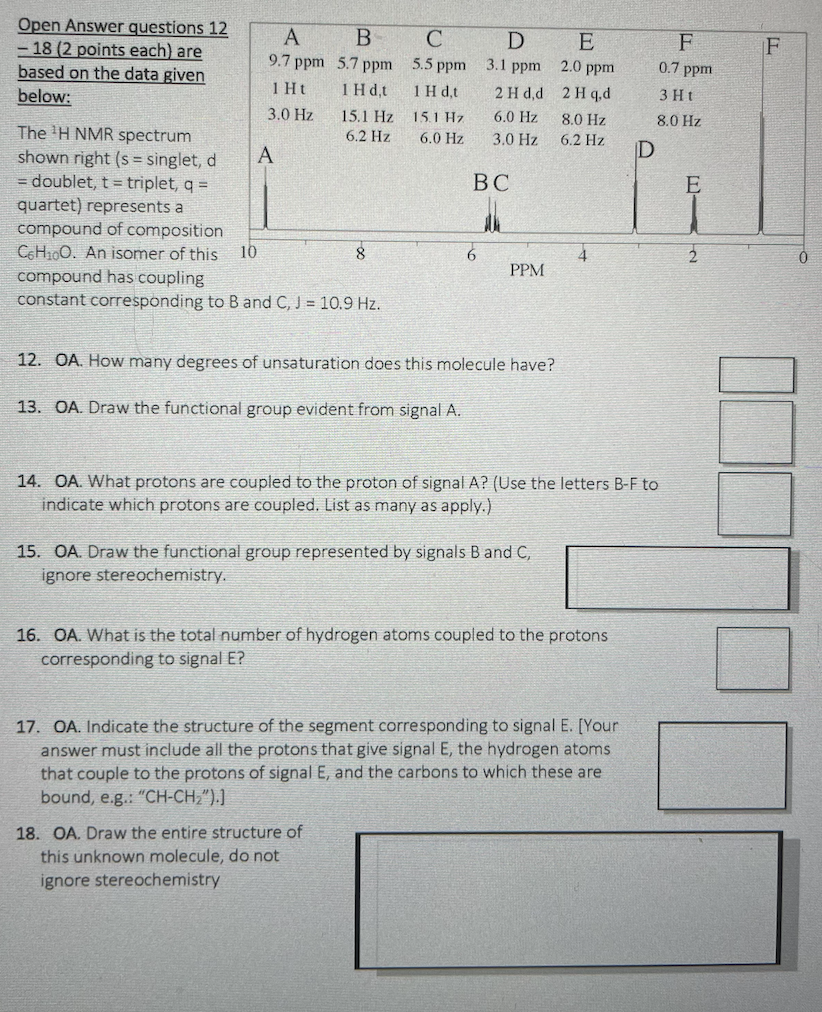
Open Answer questions 12 18 ( 2 points each ) are based on the data given below: The 2 H NMR spectrum shown right (s= singlet, d = doublet, t= triplet, q= quartet) represents a compound of composition C6H10 O. An isomer of this compound has coupling constant corresponding to B and C,J=10.9Hz. 12. OA. How many degrees of unsaturation does this molecule have? 13. OA. Draw the functional group evident from signal A. 14. OA. What protons are coupled to the proton of signal A? (Use the letters B-F to indicate which protons are coupled. List as many as apply.) 15. OA. Draw the functional group represented by signals B and C, ignore stereochemistry. 16. OA. What is the total number of hydrogen atoms coupled to the protons corresponding to signal E? 17. OA. Indicate the structure of the segment corresponding to signal E. [Your answer must include all the protons that give signal E, the hydrogen atoms that couple to the protons of signal E, and the carbons to which these are bound, e.g.: " CHCH2 ).] 18. OA. Draw the entire structure of this unknown molecule, do not ignore stereochemistry
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


