Question: options for 4, 5 , 6 are: zero, first, second Use the following information to answer questions 48. Dr. R.X.N. Raight investigates the reaction: BrO3(aq)+5Br(aq)+6H+(aq)3Br(aq)+3H2O(l)
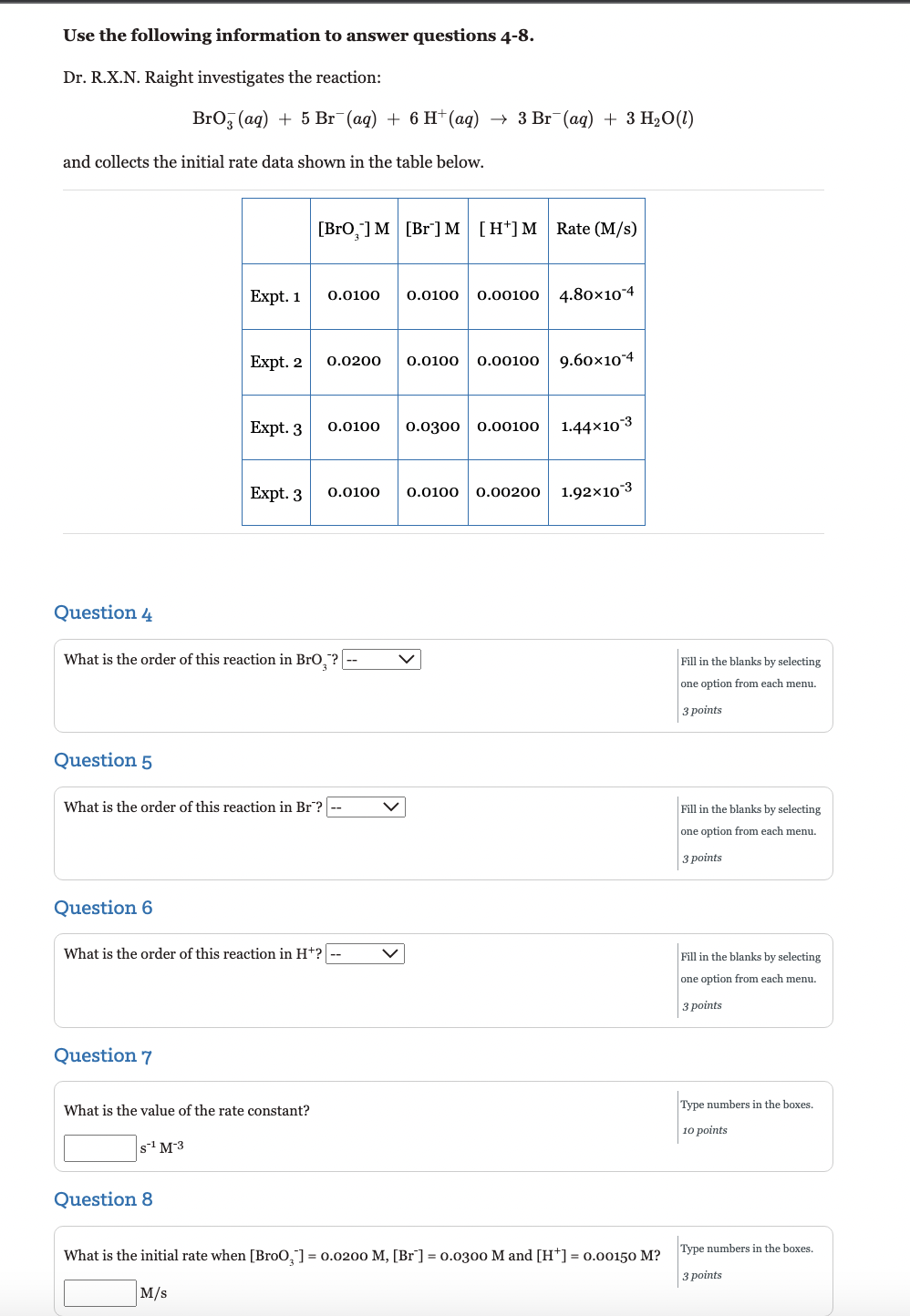
options for 4, 5 , 6 are: zero, first, second
Use the following information to answer questions 48. Dr. R.X.N. Raight investigates the reaction: BrO3(aq)+5Br(aq)+6H+(aq)3Br(aq)+3H2O(l) and collects the initial rate data shown in the table below. Question 4 What is the order of this reaction in BrO3? Fill in the blanks by selecting one option from each menu. 3 points Question 5 What is the order of this reaction in Br? Fill in the blanks by selecting one option from each menu. 3 points Question 6 What is the order of this reaction in H+? Fill in the blanks by selecting one option from each menu. 3 points Question 7 What is the value of the rate constant? s1M3 Type numbers in the boxes. lo points Question 8 What is the initial rate when [BroO3]=0.0200M,[Br]=0.0300M and [H+]=0.00150M ? Type numbers in the boxes. 3 points M/s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


