Question: options for first two are: increase, decrease, not change options for last two are.: faster, slower, the same Describe the effect of the following on
options for first two are: increase, decrease, not change
options for last two are.: faster, slower, the same
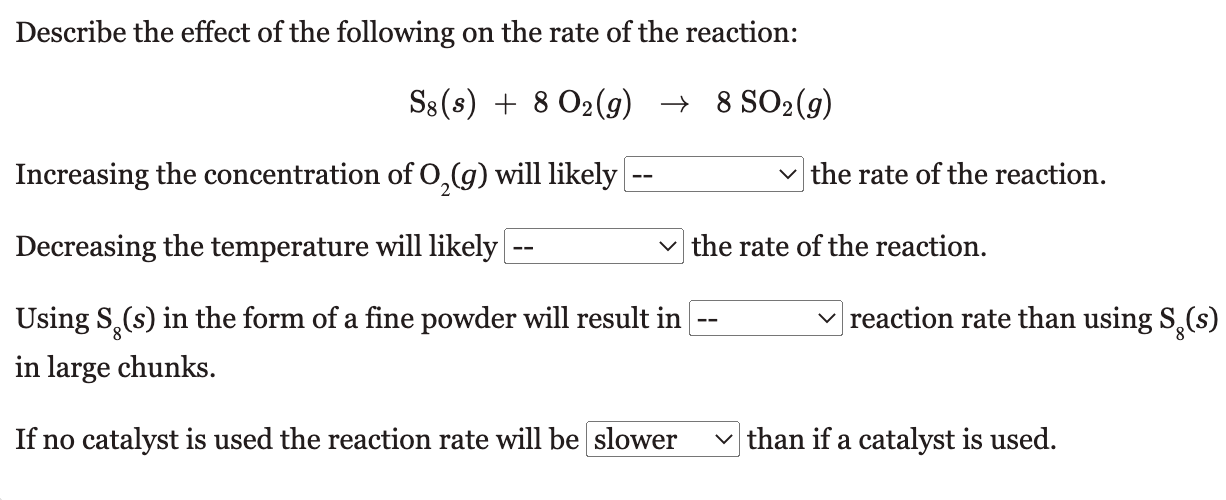
Describe the effect of the following on the rate of the reaction: S8(s)+8O2(g)8SO2(g) Increasing the concentration of O2(g) will likely the rate of the reaction. Decreasing the temperature will likely the rate of the reaction. Using S8(s) in the form of a fine powder will result in reaction rate than using S8(s) in large chunks. If no catalyst is used the reaction rate will be than if a catalyst is used
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


