Question: originally I thought it was the first answer but it is not. Thank you. In the Lewis Structure for sulfur trioxide, which of the following
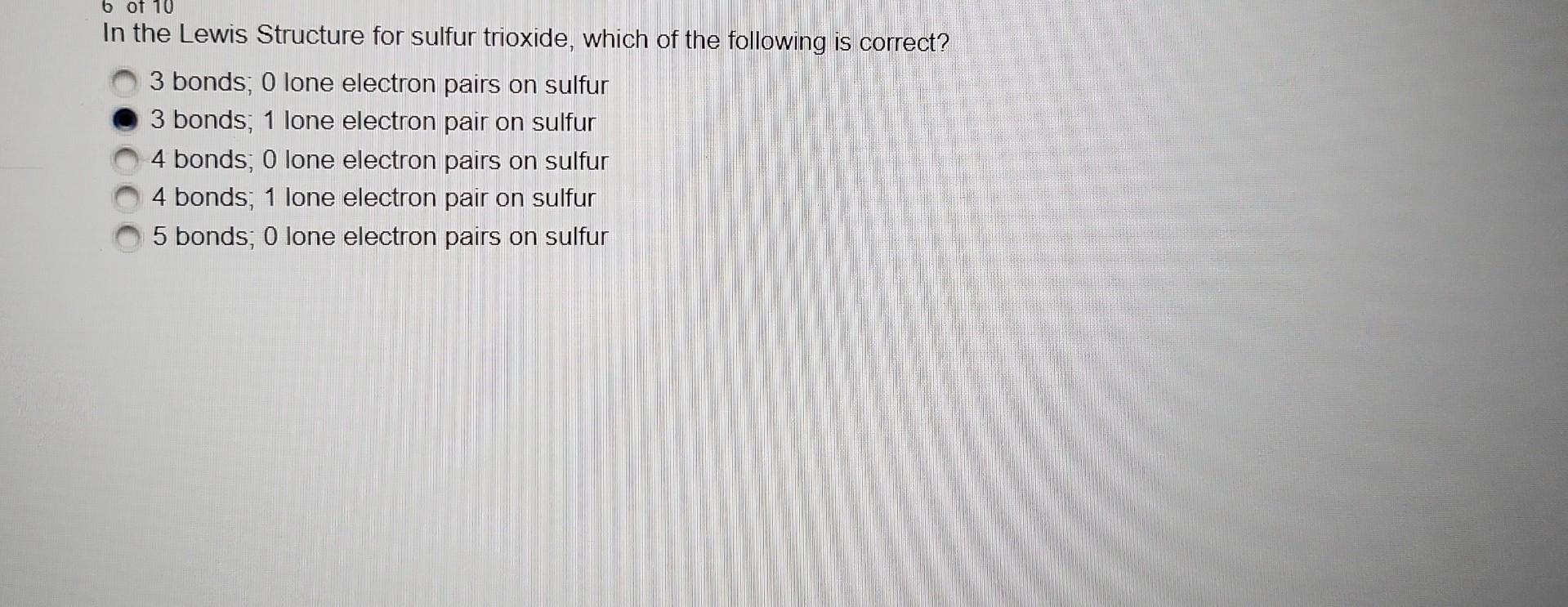
originally I thought it was the first answer but it is not. Thank you.
In the Lewis Structure for sulfur trioxide, which of the following is correct? 3 bonds; 0 lone electron pairs on sulfur 3 bonds; 1 lone electron pair on sulfur 4 bonds; 0 lone electron pairs on sulfur 4 bonds; 1 lone electron pair on sulfur 5 bonds; 0 lone electron pairs on sulfur
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


