Question: Osmosis involves a semi-permeable membrane with small pores that allow the solvent to travel across the membrane, however, larger solute molecules are unable to do
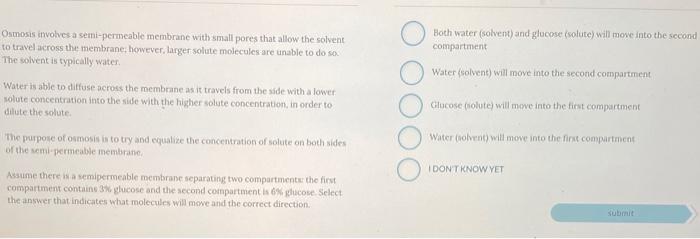
Osmosis involves a semi-permeable membrane with small pores that allow the solvent to travel across the membrane, however, larger solute molecules are unable to do so. The solvent is typically water. Both water (solvent) and glucose (solute) will move into the second compartment Water (solvent) will move into the second compartment Glucose Golute) will move into the first compartment Water is able to diffuse across the membrane as it travels from the side with a lower solute concentration into the side with the higher solute concentration, in order to dilute the solute The purpose of osmosis is to try and equalize the concentration of solute on both sides of the semi-permeable membrane Water Golvent will move into the first compartment IDONT KNOW YET Assume there is a semipermeable membrane separating two compartments the first compartment contains 3% glucose and the second compartment is glucose Select the answer that indicates what molecules will move and the correct direction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


