Question: Our engineering group have obtained the time versus conversion data listed below using a recycle Berty reactor for the hydrocracking of n-hexane: nC6H14nC4H10+C2H4 The data
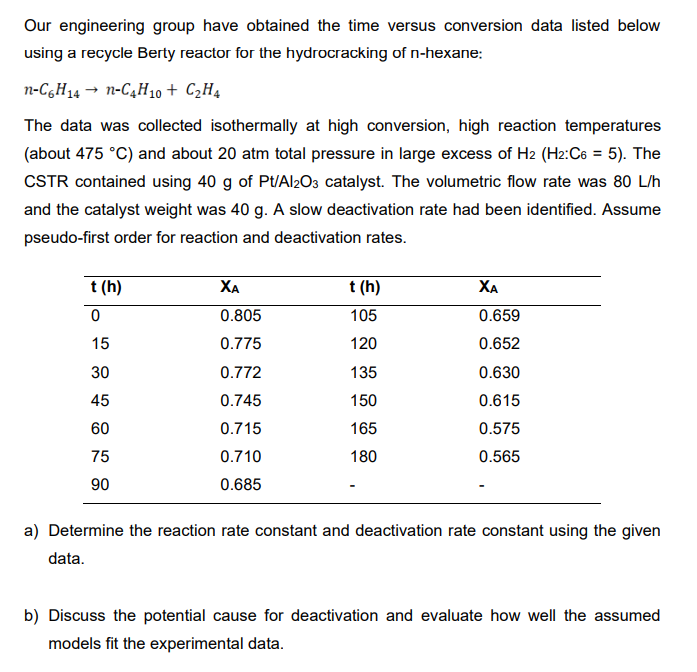
Our engineering group have obtained the time versus conversion data listed below using a recycle Berty reactor for the hydrocracking of n-hexane: nC6H14nC4H10+C2H4 The data was collected isothermally at high conversion, high reaction temperatures (about 475C ) and about 20 atm total pressure in large excess of H2(H2:C6=5). The CSTR contained using 40g of Pt/Al2O3 catalyst. The volumetric flow rate was 80L/h and the catalyst weight was 40g. A slow deactivation rate had been identified. Assume pseudo-first order for reaction and deactivation rates. a) Determine the reaction rate constant and deactivation rate constant using the given data. b) Discuss the potential cause for deactivation and evaluate how well the assumed models fit the experimental data
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


