Question: Oxygen (A) is diffusing through carbon monoxide (B) under steady-state conditions, with CO non-diffusing. The total pressure is 1105N/m2, and the temperature 0C. The partial
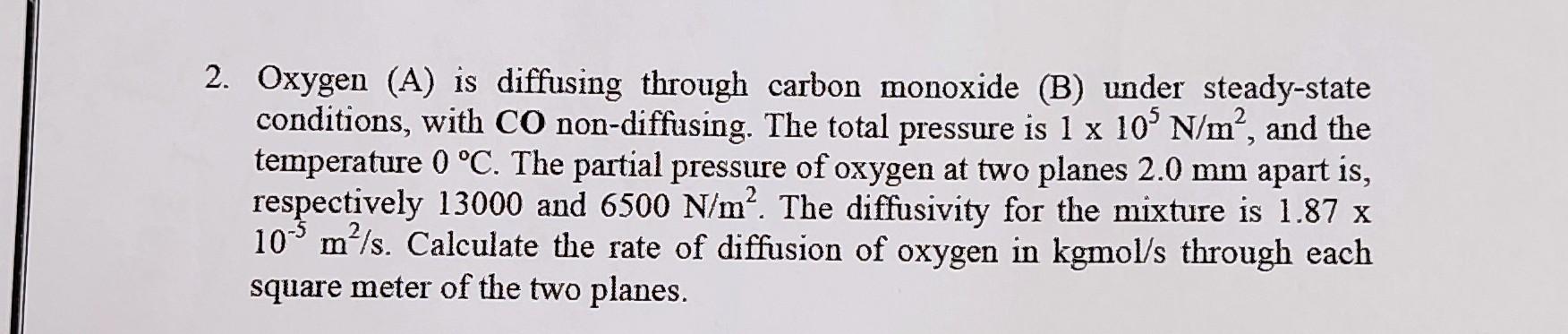
Oxygen (A) is diffusing through carbon monoxide (B) under steady-state conditions, with CO non-diffusing. The total pressure is 1105N/m2, and the temperature 0C. The partial pressure of oxygen at two planes 2.0mm apart is, respectively 13000 and 6500N/m2. The diffusivity for the mixture is 1.87x 105m2/s. Calculate the rate of diffusion of oxygen in kgmol/s through each square meter of the two planes
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


