Question: Oxygen and Nitrogen diffuse in a counter-current ideal gas manner through a 0.0049m diameter circular channel of length 0.0101m at a temperature of 298K and
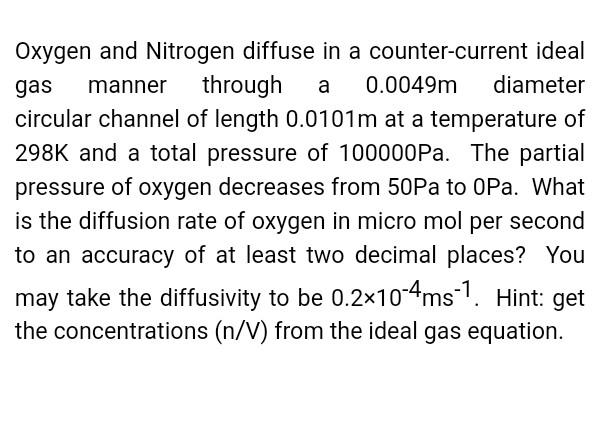
Oxygen and Nitrogen diffuse in a counter-current ideal gas manner through a 0.0049m diameter circular channel of length 0.0101m at a temperature of 298K and a total pressure of 100000Pa. The partial pressure of oxygen decreases from 50Pa to OPa. What is the diffusion rate of oxygen in micro mol per second to an accuracy of at least two decimal places? You may take the diffusivity to be 0.2104ms1. Hint: get the concentrations (n/V) from the ideal gas equation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


