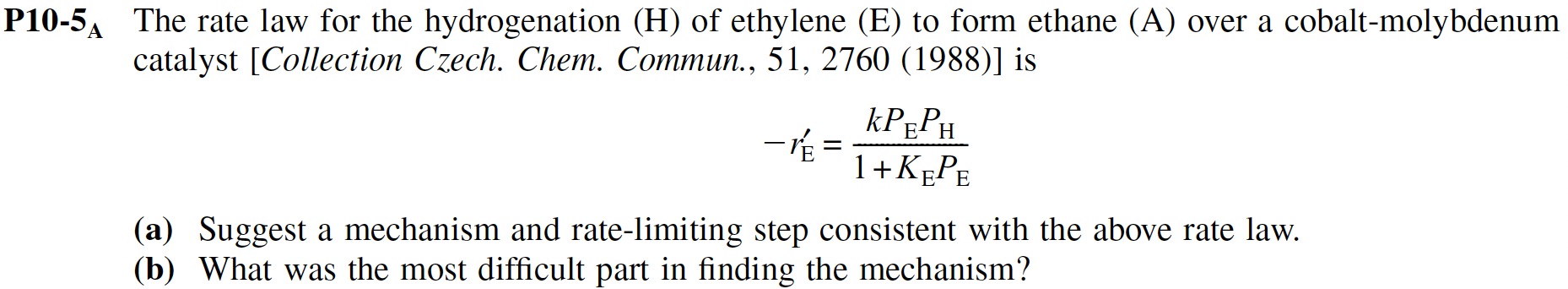
Question: P 1 0 - 5 ? A The rate law for the hydrogenation ( H ) of ethylene ( E ) to form ethane (
P The rate law for the hydrogenation of ethylene to form ethane over a cobaltmolybdenum
catalyst Collection Czech. Chem. Commun., is
a Suggest a mechanism and ratelimiting step consistent with the above rate law.
b What was the most difficult part in finding the mechanism?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


