Question: p Please Show All the Necessary steps for the material balance thank you 5. Mass balance around reactor (10 points) The fresh feed to an
 p
p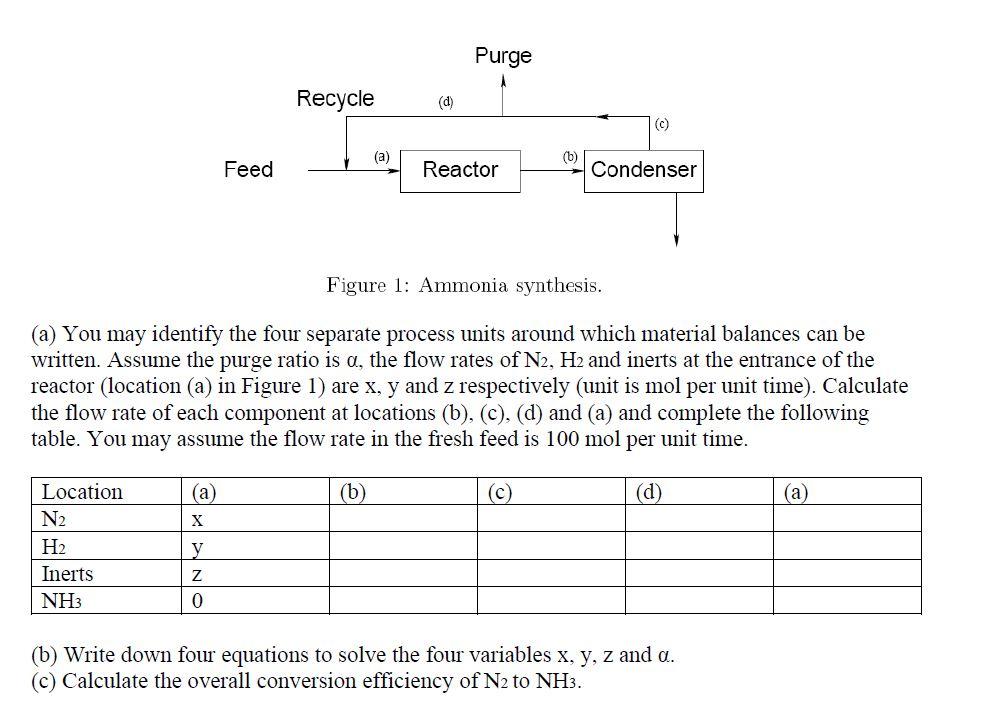
Please Show All the Necessary steps for the material balance
thank you
5. Mass balance around reactor (10 points) The fresh feed to an ammonia production process contains 24.75 mole% N2, 74.25 mole% H2 and the balance inerts. The feed is combined with a recycle stream containing the same species and the combined stream is fed to a reactor in which a 25% single pass conversion of nitrogen is achieved. The products pass through a condenser in which all of the ammonia is removed and the remaining gases are recycled. To prevent buildup of the inerts, a purge stream must be taken off. The recycle stream contains 12.5 mole% inerts. Purge Recycle (d) (c) (a) (b) Feed Reactor Condenser Figure 1: Ammonia synthesis. (a) You may identify the four separate process units around which material balances can be written. Assume the purge ratio is a, the flow rates of N2, H2 and inerts at the entrance of the reactor (location (a) in Figure 1) are x, y and z respectively (unit is mol per unit time). Calculate the flow rate of each component at locations (b), (c), (d) and (a) and complete the following table. You may assume the flow rate in the fresh feed is 100 mol per unit time. (a) (b) (c) (d) (a) X Location N2 H2 Inerts NH3 Z 0 (b) Write down four equations to solve the four variables x, y, z and o.. (c) Calculate the overall conversion efficiency of N2 to NH3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


