Question: Paper Homework 5 ( to be turned in before class on Friday, September 2 7 ) The picture below shows the cycle of an engine
Paper Homework to be turned in before class on Friday, September
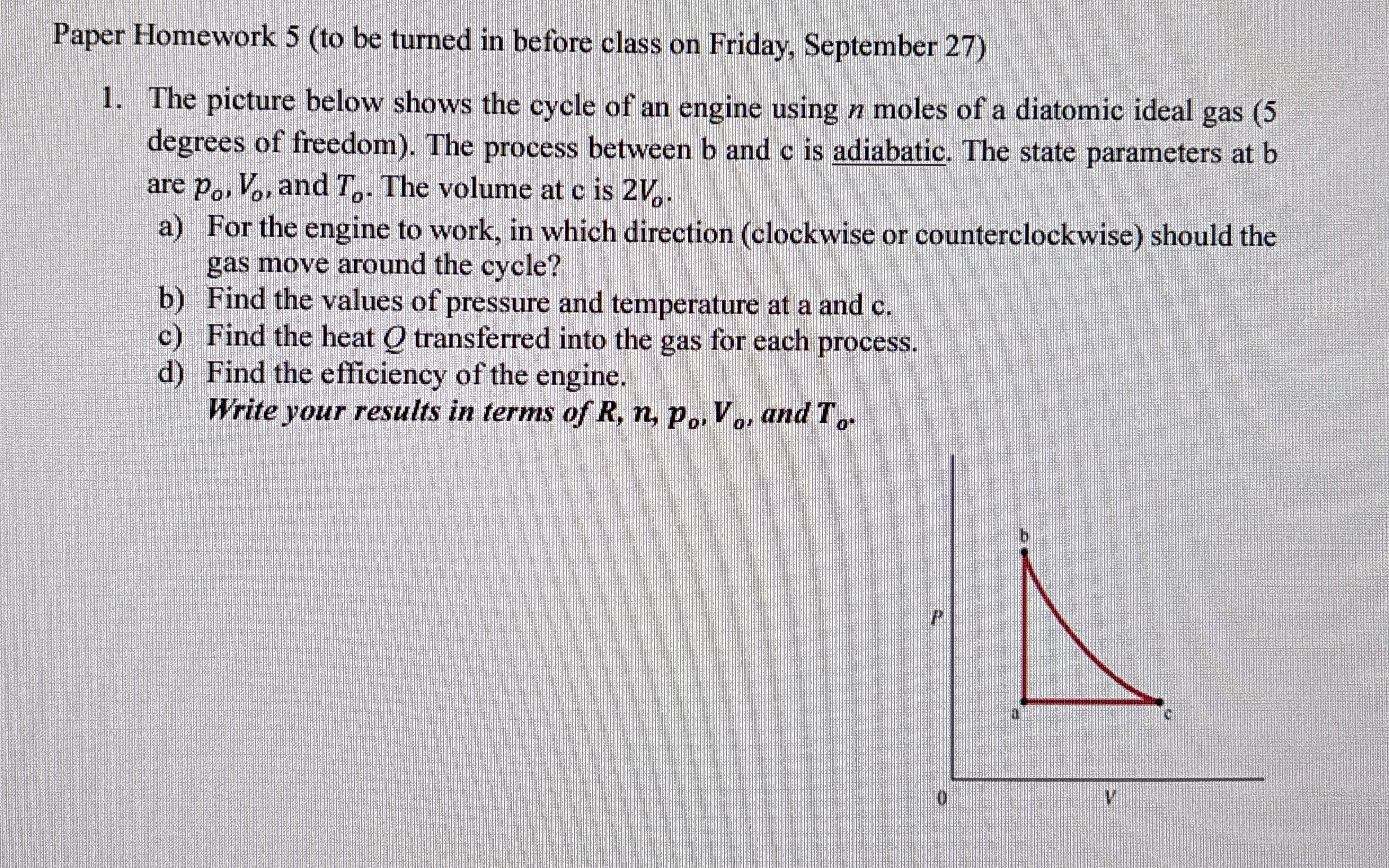
The picture below shows the cycle of an engine using moles of a diatomic ideal gas degrees of freedom The process between b and c is adiabatic. The state parameters at b are and The volume at c is
a For the engine to work, in which direction clockwise or counterclockwise should the gas move around the cycle?
b Find the values of pressure and temperature at a and c
c Find the heat transferred into the gas for each process.
d Find the efficiency of the engine.
Write your results in terms of and
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


