Question: part 1 part 2 At a particular temperature, the equilibrium constant is 2.1x10^3 for the synthesis of hydrogen fluoride from its elements: H2(g) + F2(g)
part 1 

part 2
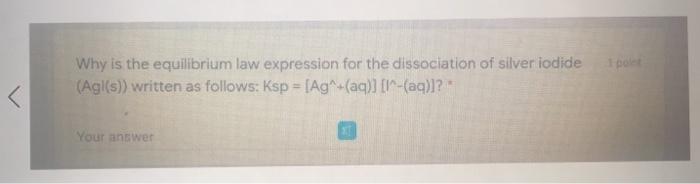
At a particular temperature, the equilibrium constant is 2.1x10^3 for the synthesis of hydrogen fluoride from its elements: H2(g) + F2(g) = 2 HF(g). Determine how the chemical system will behave when [H2(g)] = 2.2x10^-2 mol/L: (F2(g)] - 2.2x10^-3 mol/L: (HF(g) = 9.6x10^-2 mol/L. shift lett O shift right O no change polet Why is the equilibrium law expression for the dissociation of silver iodide (Agl(s)) written as follows: Ksp = [Ag (aq)] -(aq)]? Your
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


