Question: Part 4: Percent Error Calculations: In lab class, we will be using the % error to determine the accuracy of our lab results. Here is
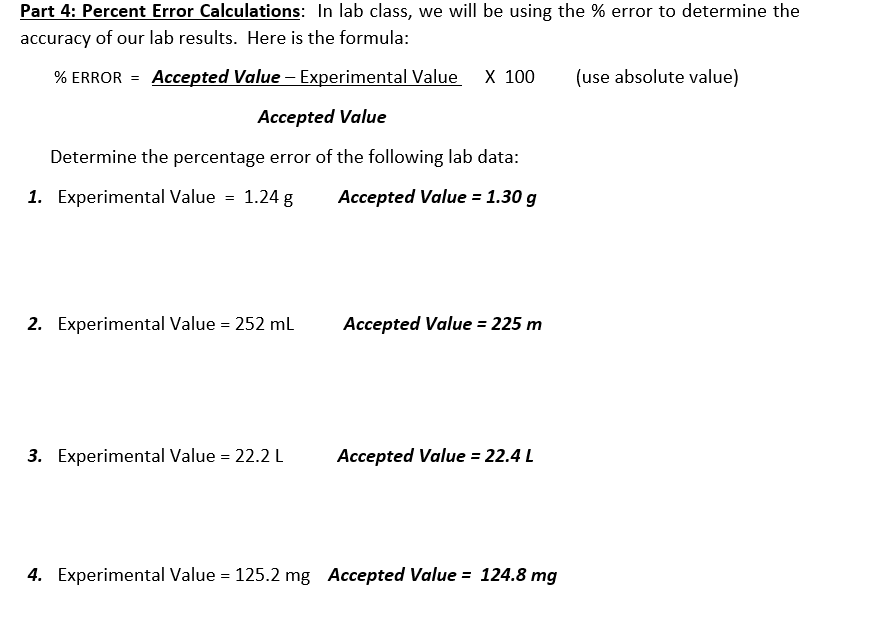
Part 4: Percent Error Calculations: In lab class, we will be using the \% error to determine the accuracy of our lab results. Here is the formula: % ERROR = Accepted Value Experimental Value X 100 (use absolute value) Accepted Value Determine the percentage error of the following lab data: 1. Experimental Value =1.24g Accepted Value =1.30g 2. Experimental Value =252mL Accepted Value =225m 3. Experimental Value =22.2L Accepted Value =22.4L 4. Experimental Value =125.2mg Accepted Value =124.8mg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


