Question: Part 4: Solution Phase Stoichiometry - Combining Density and Percent Composition 5. A hydrochloric acid solution consists of 28.0% HCl by mass, and has a
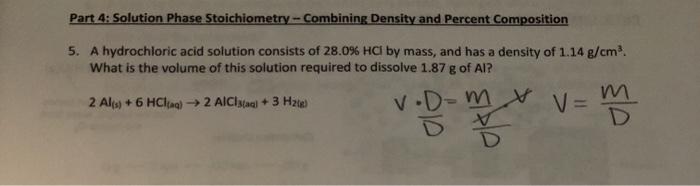
Part 4: Solution Phase Stoichiometry - Combining Density and Percent Composition 5. A hydrochloric acid solution consists of 28.0% HCl by mass, and has a density of 1.14 g/cm What is the volume of this solution required to dissolve 1.87 g of Al? 2 Al(s) + 6 HCl) 2 AlCal + 3 H2) V.Dom & V= m V mm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


