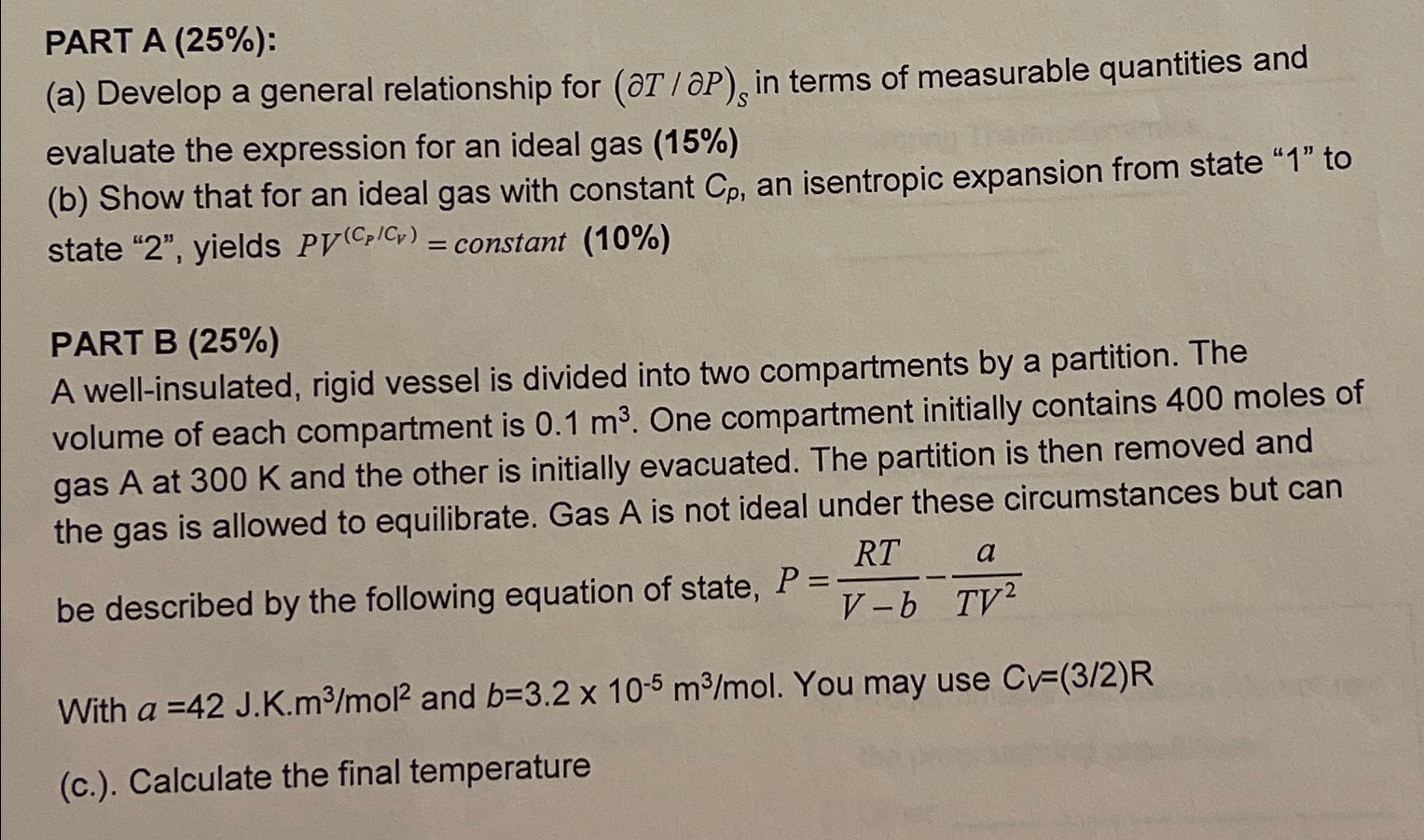
Question: PART A ( 2 5 % ) : ( a ) Develop a general relationship for ( d e l T d elP ) S
PART A :
a Develop a general relationship for elP in terms of measurable quantities and evaluate the expression for an ideal gas
b Show that for an ideal gas with constant an isentropic expansion from state to state yields constant
PART B
A wellinsulated, rigid vessel is divided into two compartments by a partition. The volume of each compartment is One compartment initially contains moles of gas at and the other is initially evacuated. The partition is then removed and the gas is allowed to equilibrate. Gas is not ideal under these circumstances but can be described by the following equation of state,
With and You may use
c Calculate the final temperature
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


