Question: Part A A certain first-order reaction has a rate constant of 2.20x10-25-1 at 24 C. What is the value of k at 69 C if
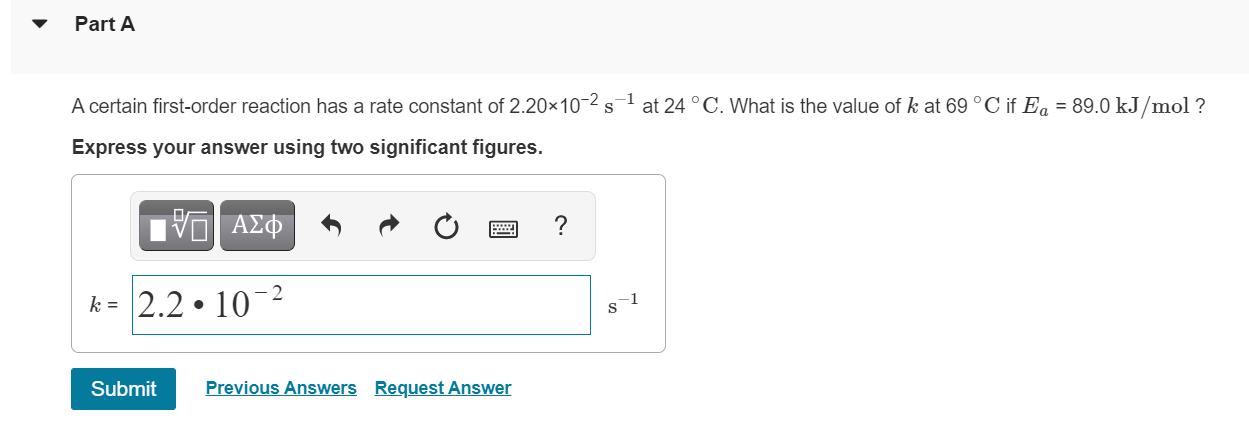
Part A A certain first-order reaction has a rate constant of 2.20x10-25-1 at 24 C. What is the value of k at 69 C if Ea = 89.0 kJ/mol ? Express your answer using two significant figures. V AEO k= 2.2 10-2 1 s Submit Previous Answers Request
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


