Question: Part A An electrolytic process oxidizes nickel sulfide in a two-step reaction: NizS2(s) + Ni2+ (aq) + 2NiS(s) + 2e 2NiS(s) 2Ni2+ (aq) + 2S(s)
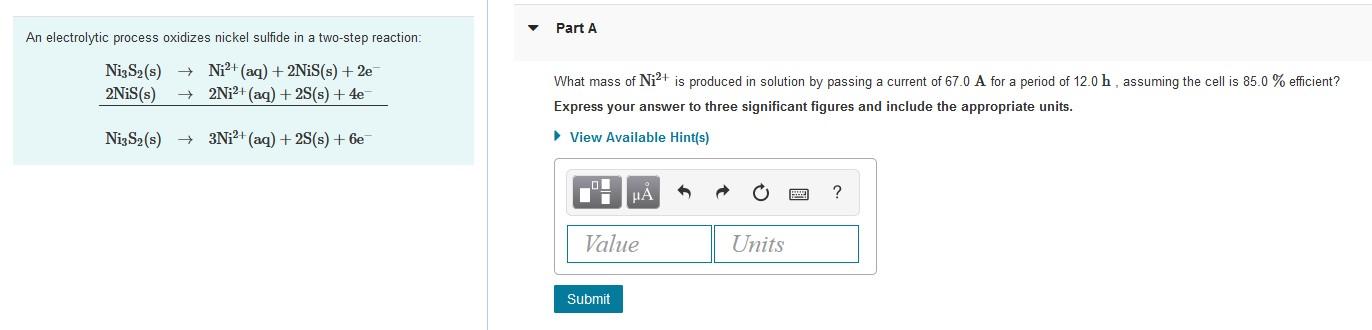
Part A An electrolytic process oxidizes nickel sulfide in a two-step reaction: NizS2(s) + Ni2+ (aq) + 2NiS(s) + 2e 2NiS(s) 2Ni2+ (aq) + 2S(s) + 4e What mass of Ni2+ is produced in solution by passing a current of 67.0 A for a period of 12.0 h, assuming the cell is 85.0 % efficient? Express your answer to three significant figures and include the appropriate units. NizS2(s) + 3Ni2+ (aq) + 2S(s) + 6e View Available Hint(s) ? Value Units Submit
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


