Question: Part (a) and (b) only! Skip if u do not know Consider energy transfer in a stationary fluid or solid. The internal e ergy per
Part (a) and (b) only! Skip if u do not know
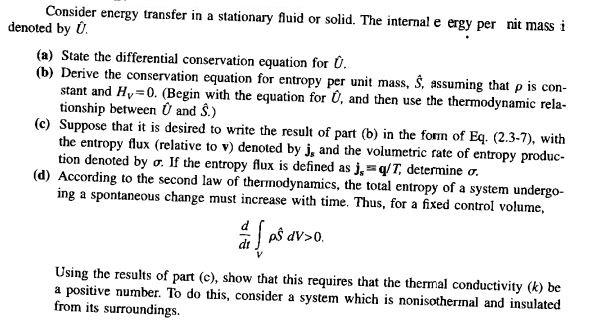
Consider energy transfer in a stationary fluid or solid. The internal e ergy per nit mass i denoted by 0. (a) State the differential conservation equation for U. (b) Derive the conservation equation for entropy per unit mass, S, assuming that p is con- stant and Hy=0. (Begin with the equation for 0, and then use the thermodynamic rela- tionship between 0 and S.) (e) Suppose that it is desired to write the result of part (b) in the form of Eq. (2.3-7), with the entropy flux (relative to v) denoted by j, and the volumetric rate of entropy produc- tion denoted by o. If the entropy flux is defined as js =qT, determine o. (d) According to the second law of thermodynamics, the total entropy of a system undergo- ing a spontaneous change must increase with time. Thus, for a fixed control volume, d as dV>0. a las Using the results of part (e), show that this requires that the thermal conductivity (k) be a positive number. To do this, consider a system which is nonisothermal and insulated from its surroundings. Consider energy transfer in a stationary fluid or solid. The internal e ergy per nit mass i denoted by 0. (a) State the differential conservation equation for U. (b) Derive the conservation equation for entropy per unit mass, S, assuming that p is con- stant and Hy=0. (Begin with the equation for 0, and then use the thermodynamic rela- tionship between 0 and S.) (e) Suppose that it is desired to write the result of part (b) in the form of Eq. (2.3-7), with the entropy flux (relative to v) denoted by j, and the volumetric rate of entropy produc- tion denoted by o. If the entropy flux is defined as js =qT, determine o. (d) According to the second law of thermodynamics, the total entropy of a system undergo- ing a spontaneous change must increase with time. Thus, for a fixed control volume, d as dV>0. a las Using the results of part (e), show that this requires that the thermal conductivity (k) be a positive number. To do this, consider a system which is nonisothermal and insulated from its surroundings
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


