Question: Part A . Determine the rate at which CO2 is consumed as it reacts with water to form gas hydrate. Hint: R= dn/dt. Part B.
Part A. Determine the rate at which CO2 is consumed as it reacts with water to form gas hydrate. Hint: R= dn/dt.
Part B. What is the initial rate (R at t = 0) of hydrate formation under these conditions?
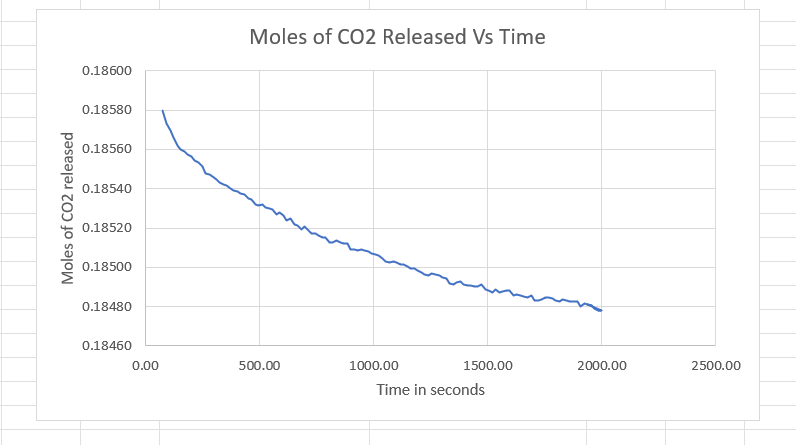
Moles of CO2 Released Vs Time 0.18600 0.18580 0.18560 0.18540 Moles of CO2 released 0.18520 0.18500 0.18480 0.18460 0.00 500.00 2000.00 2500.00 1000.00 1500.00 Time in seconds
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


