Question: 1. If a reaction mixture initially contains a CO concentration of 0.1510 M and a Cl2 concentration of 0.179 M at 1000 K. What is
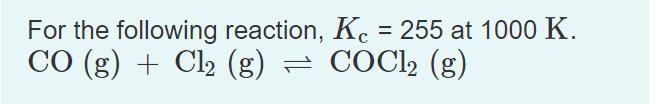
1. If a reaction mixture initially contains a CO concentration of 0.1510 M and a Cl2 concentration of 0.179 M at 1000 K. What is the equilibrium concentration of CO at 1000 K?
2. What is the equilibrium concentration of Cl2 at 1000 K?
3. What is the equilibrium concentration of COCl2 at 1000 K?
For the following reaction, Kc = 255 at 1000 K. CO (g) + Cl (g) = COCl (g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


