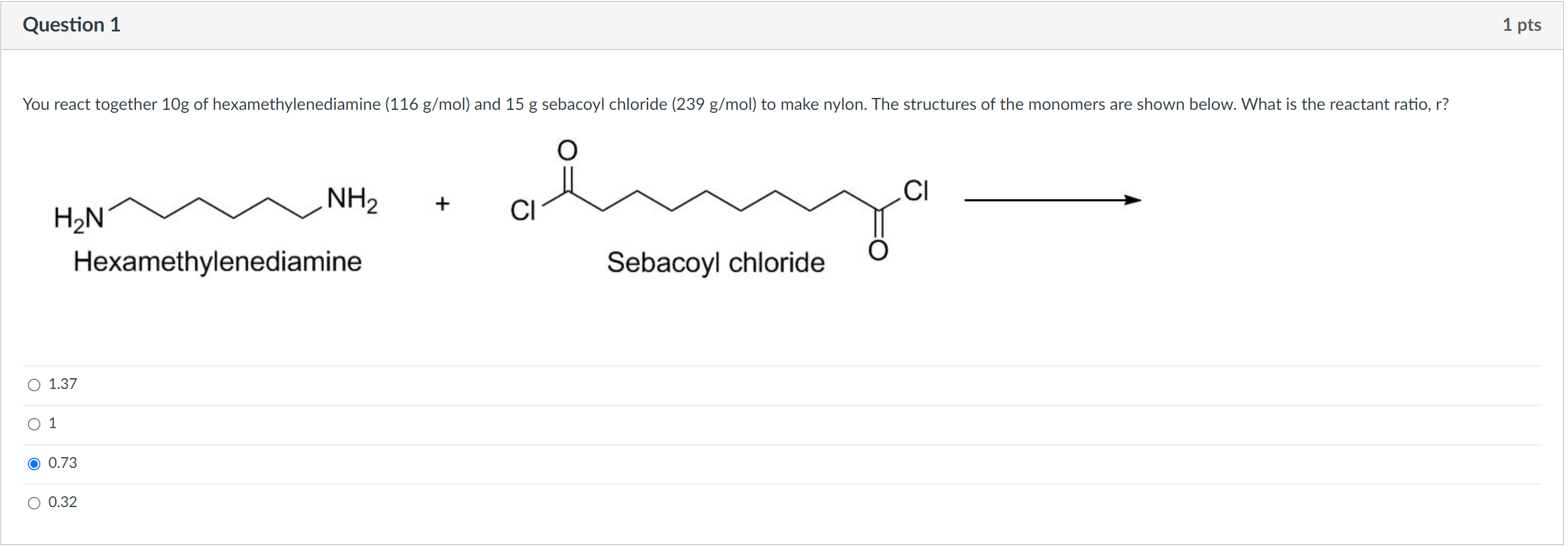
Question: part a is 0.73. how do i get part b? Question 1 1 pts You react together 10g of hexamethylenediamine (116 g/mol) and 15 g
 part a is 0.73. how do i get part b?
part a is 0.73. how do i get part b?
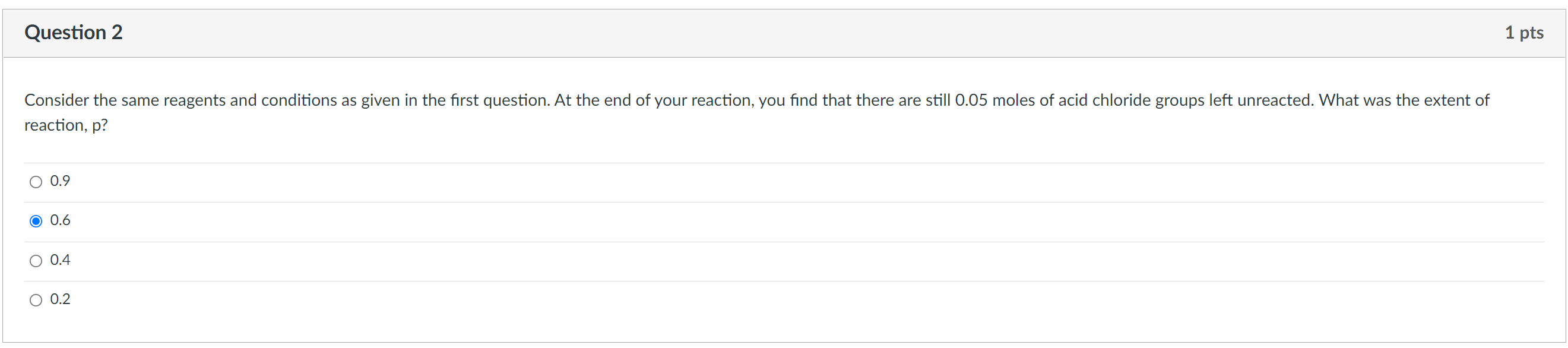
Question 1 1 pts You react together 10g of hexamethylenediamine (116 g/mol) and 15 g sebacoyl chloride (239 g/mol) to make nylon. The structures of the monomers are shown below. What is the reactant ratio, r? CI + CI NH2 H2N Hexamethylenediamine Sebacoyl chloride O 1.37 1 0.73 O 0.32 Question 2 1 pts Consider the same reagents and conditions as given in the first question. At the end of your reaction, you find that there are still 0.05 moles of acid chloride groups left unreacted. What was the extent of reaction, p? 0.9 O 0.6 0.4 0.2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


