Question: Part A Part 8 If each dot represents a concentration of 0.10M, what is the rate of the reaction AB between 0 and 20 s?
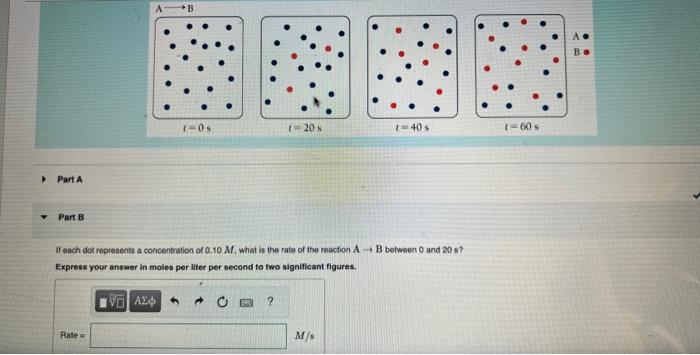
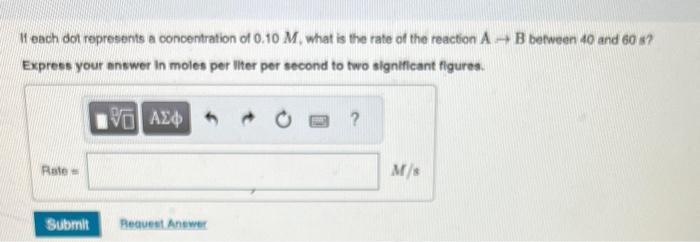
Part A Part 8 If each dot represents a concentration of 0.10M, what is the rate of the reaction AB between 0 and 20 s? Express your answer in moles per liter per second to two significant figures. It each dot represents a concentration of 0.10M, what is the rate of the reaction AB befween 40 and 60 s? Exprees your answer in moles per Iliter per second to two significant flgures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


