Question: Part A Ti+ Write The Valence-Shell Ground-State Electron Configuration For Each Of The Following Species. Type The Valence-Shell Ground-State Electron Configuration In The Spdf Notation
Part A Ti+ Write The Valence-Shell Ground-State Electron Configuration For Each Of The Following Species. Type The Valence-Shell Ground-State Electron Configuration In The Spdf Notation Following The Format Given In The Examples Below. All The Sublevels In The Valence-Shell Of The Element Should Be Included In The Answer Ifa Valence Shell Sublevel Is Empty,
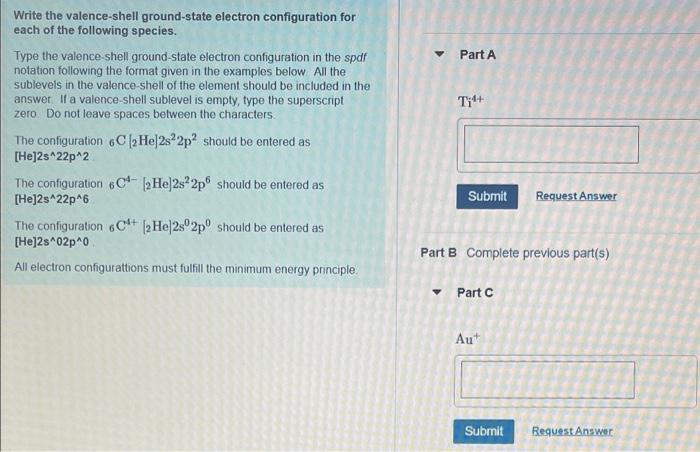
Write the valence-shell ground-state electron configuration for each of the following species. Type the valence-shell ground-state electron configuration in the spdf notation following the format given in the examples below. All the sublevels in the valence-shell of the element should be included in the answer. If a valence-shell sublevel is empty, type the superscript zero. Do not leave spaces between the characters. The configuration 6C 2 Hel2s22p2 should be entered as [He]2s^22p^2 The configuration 6C 2 He]2s22p should be entered as [He]2s^22p^6 The configuration 6C4+ [2Hel2s02p0 should be entered as [He]2s^02p^0 All electron configurattions must fulfill the minimum energy principle. Part A Ti4+ Submit Request Answer Part B Complete previous part(s) Part C Au+ Submit Request Answer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


