Question: Part A Use these data to determine the Michaelis constant for invertase. The rate of reaction can be determined by measuring the change in optical
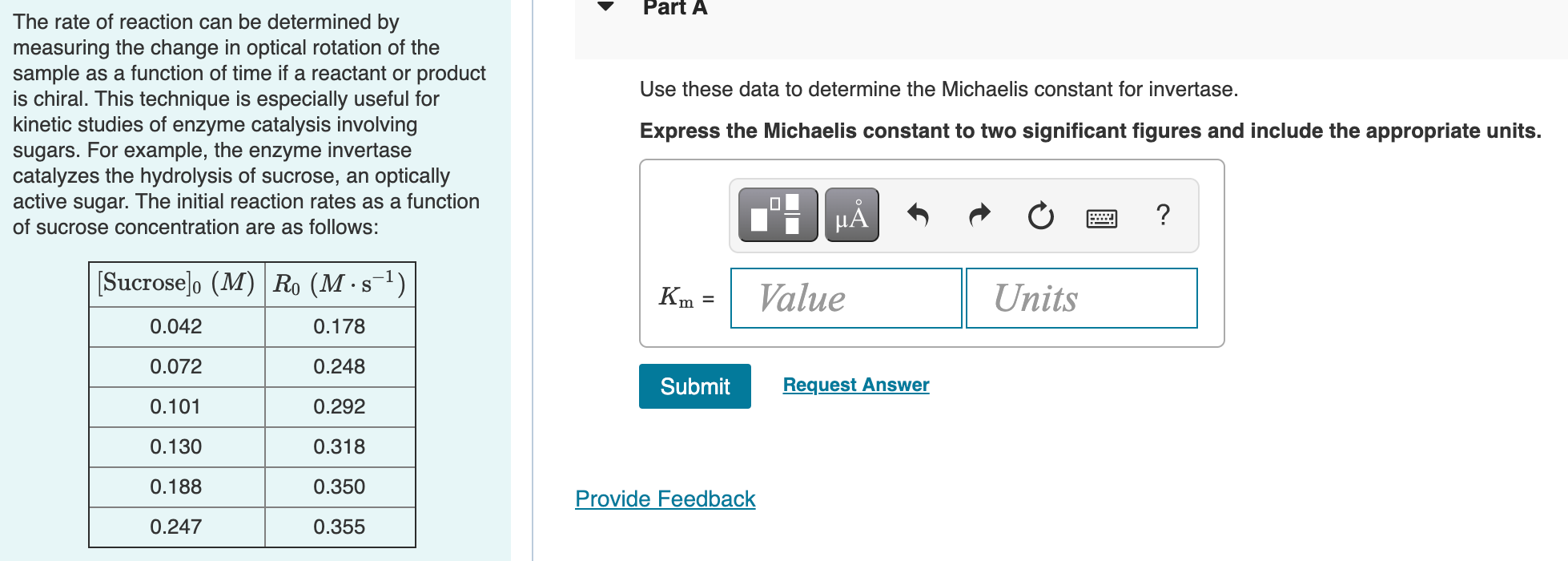
Part A Use these data to determine the Michaelis constant for invertase. The rate of reaction can be determined by measuring the change in optical rotation of the sample as a function of time if a reactant or product is chiral. This technique is especially useful for kinetic studies of enzyme catalysis involving sugars. For example, the enzyme invertase catalyzes the hydrolysis of sucrose, an optically active sugar. The initial reaction rates as a function of sucrose concentration are as follows: Express the Michaelis constant to two significant figures and include the appropriate units. H ? [Sucrose]. (M) R. (Ms-1) .S Km Value Units 0.042 0.178 0.072 0.248 Submit Request Answer 0.101 0.292 0.130 0.318 0.188 0.350 Provide Feedback 0.247 0.355
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


