Question: Part A Which has the smallest dipole-dipole forces? CH3 CI HCI AsH3 CO Submit Request Answer Part A Choose the molecule or compound that exhibits
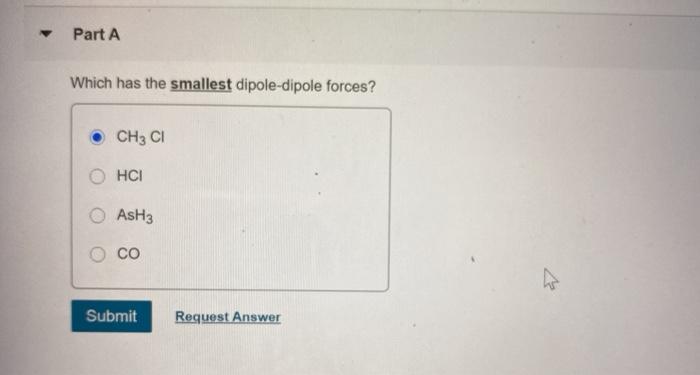
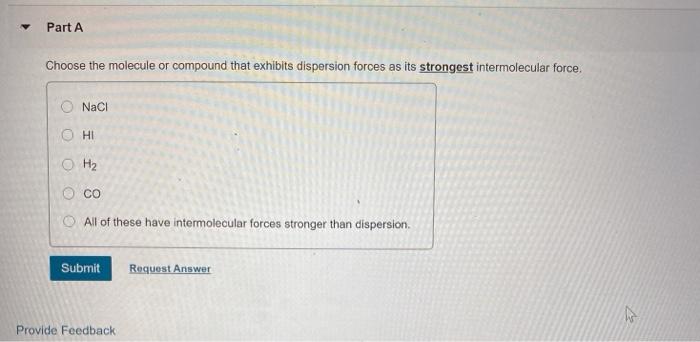
Part A Which has the smallest dipole-dipole forces? CH3 CI HCI AsH3 CO Submit Request Answer Part A Choose the molecule or compound that exhibits dispersion forces as its strongest intermolecular force. NaCl O H2 All of these have intermolecular forces stronger than dispersion Submit Request Answer Provide Feedback
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


