Question: Part b: Molecular Geometry In this experiment you will apply valence shell electron pair repulsion theory (VSEPR) to predict molecular geometry for compounds you chose
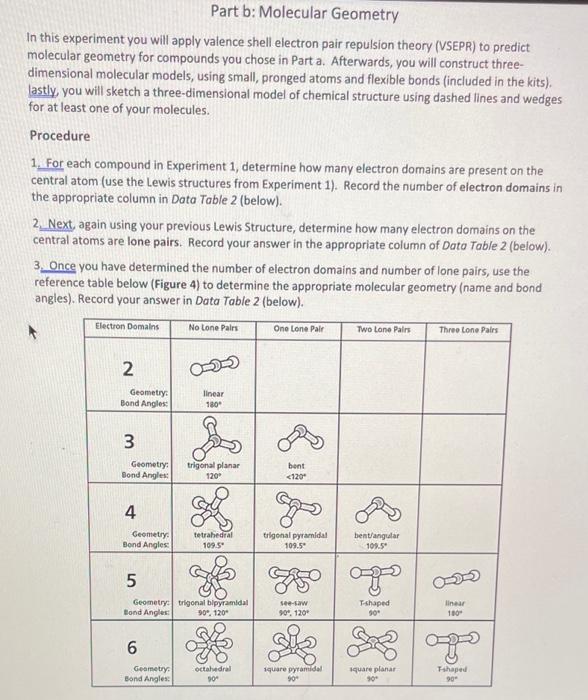
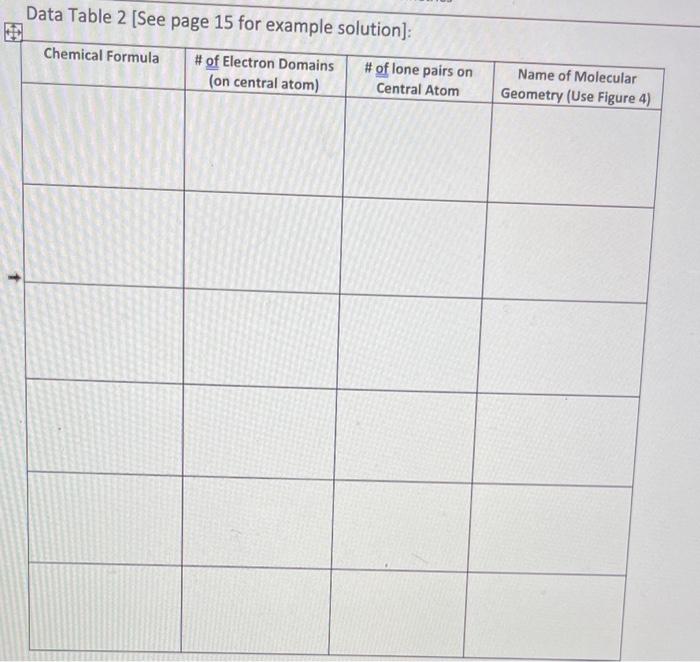
Part b: Molecular Geometry In this experiment you will apply valence shell electron pair repulsion theory (VSEPR) to predict molecular geometry for compounds you chose in Part a. Afterwards, you will construct threedimensional molecular models, using small, pronged atoms and flexible bonds (included in the kits). lastly you will sketch a three-dimensional model of chemical structure using dashed lines and wedges for at least one your molecules. Procedure 1. For each compound in Experiment 1, determine how many electron domains are present on the central atom (use the Lewis structures from Experiment 1). Record the number of electron domains in the appropriate column in Data Table 2 (below). 2. Next, again using your previous Lewis Structure, determine how many electron domains on the central atoms are lone pairs. Record your answer in the appropriate column of Dato Table 2 (below). 3. Once you have determined the number of electron domains and number of lone pairs, use the reference table below (Figure 4 ) to determine the appropriate molecular geometry (name and bond angles). Record your answer in Data Table 2 (below). Data Table 2 [See page 15 for pxamnla onl...t:-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


