Question: Part C - Generic Phase Diagram. - Answer the questions below in relation to the following generic phase diagram. 73 5. Which section represents the
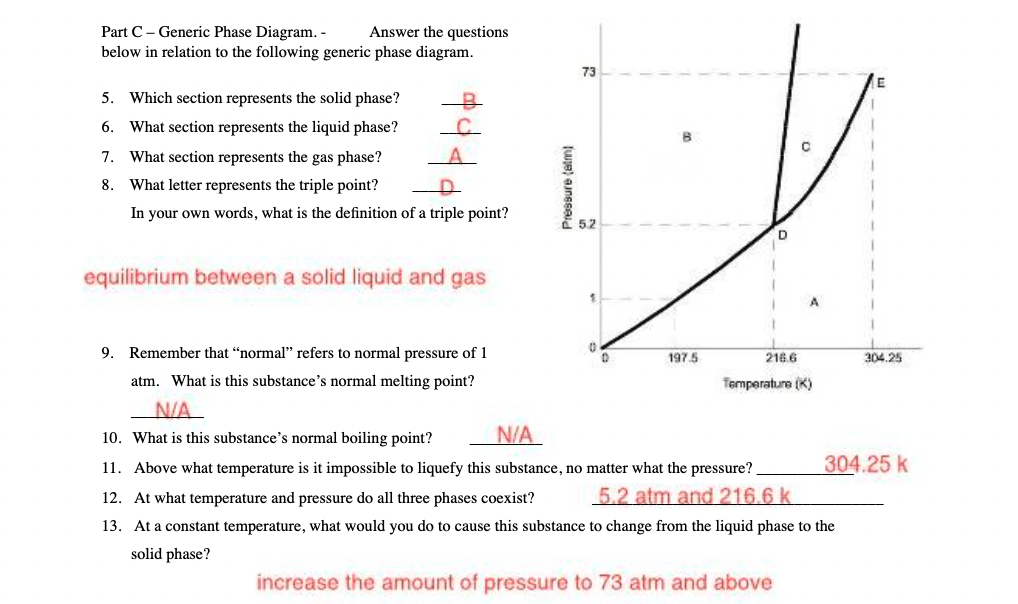
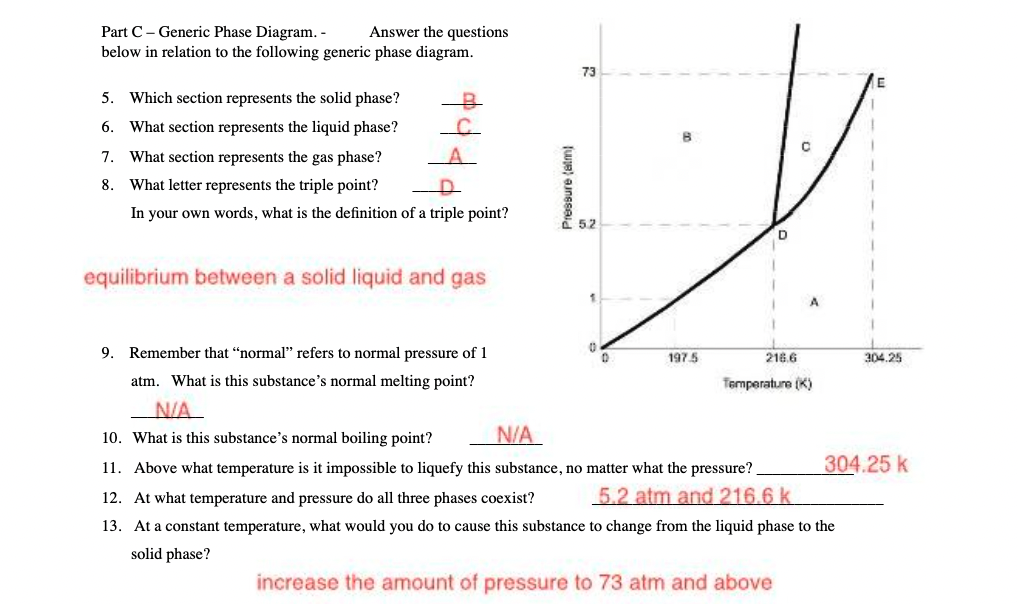
Part C - Generic Phase Diagram. - Answer the questions below in relation to the following generic phase diagram. 73 5. Which section represents the solid phase? -8 6. 'What section represents the liquid phase? o 7. What section represents the gas phase? i g 8. 'What letter represents the triple point? ey g In your own words, what is the definition of a triple point? g . equilibrium between a solid liquid and gas 3 9. Remember that \"normal\" refers to normal pressure of 1 0 o 197 5 ME6 e 2% atm. What is this substance's normal melting point? Temparature () N/A 10. What is this substance's normal boiling point? N/A 11. Above what temperature is it impossible to liquefy this substance, no matter what the pressure? 304.25 k 12. At what temperature and pressure do all three phases coexist? 5 2 {:"f] amd 2 186 f} k 13. At a constant temperature, what would you do to cause this substance to change from the liquid phase to the solid phase? increase the amount of pressure to 73 atm and above
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


