Question: PART C Identifying a Reactant TABLE 2.3 Observations of Reactant 2 Observation Measurement Mass of empty crucible and lid 8 31.38 32.37 Mass of crucible,
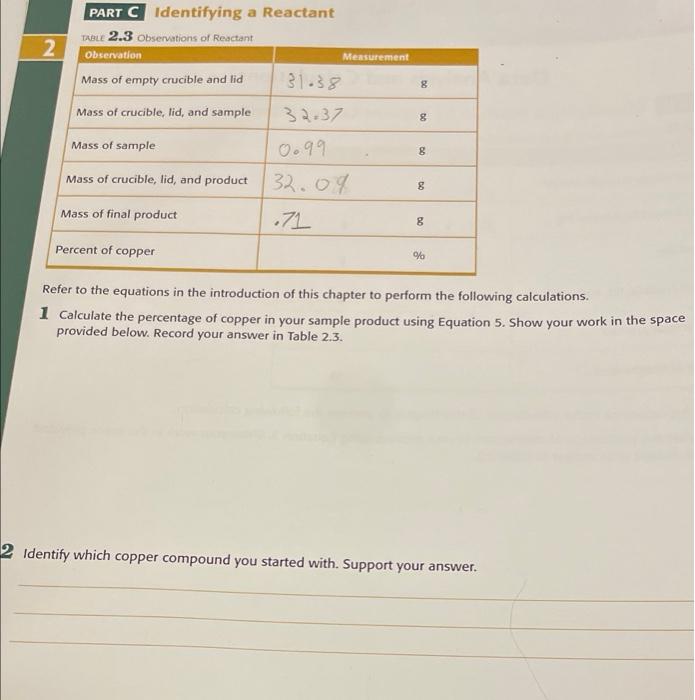
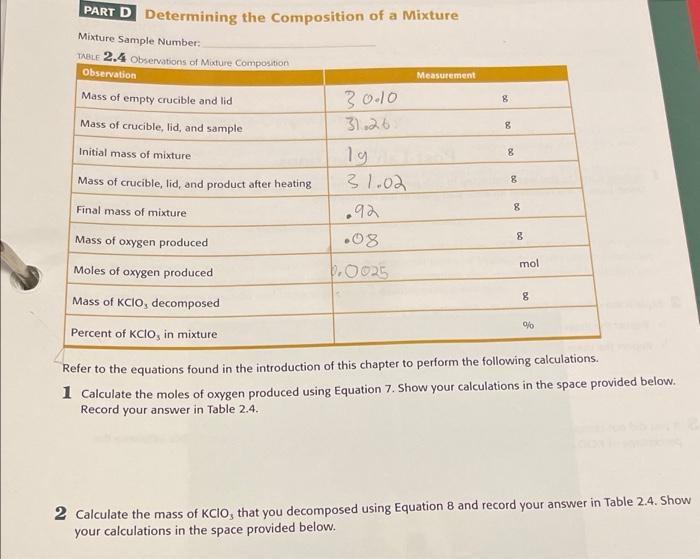



PART C Identifying a Reactant TABLE 2.3 Observations of Reactant 2 Observation Measurement Mass of empty crucible and lid 8 31.38 32.37 Mass of crucible, lid, and sample g g Mass of sample 0.99 Mass of crucible, lid, and product 32.09 Mass of final product .71 8 8 Percent of copper % Refer to the equations in the introduction of this chapter to perform the following calculations. 1 Calculate the percentage of copper in your sample product using Equation 5. Show your work in the space provided below. Record your answer in Table 2.3. 2 Identify which copper compound you started with. Support your answer. PART D Determining the Composition of a Mixture Mixture Sample Number: TABLE 2.4 Observations of Mature Composition Observation Measurement 8 8 Mass of empty crucible and lid Mass of crucible, lid, and sample Initial mass of mixture Mass of crucible, lid, and product after heating Final mass of mixture 8 30.10 31.26 19 31.02 .92 .08 8 8 8 Mass of oxygen produced mol Moles of oxygen produced .0025 8 Mass of KCIO, decomposed Percent of KClo, in mixture Refer to the equations found in the introduction of this chapter to perform the following calculations. 1 Calculate the moles of oxygen produced using Equation 7. Show your calculations in the space provided below. Record your answer in Table 2.4. 2 Calculate the mass of KCIO, that you decomposed using Equation 8 and record your answer in Table 2.4. Show your calculations in the space provided below. Post-Lab Questions 1 In Parts Cand D, you were instructed to only partially cover the crucible with the lid. Why was this necessary? (Hint: Look at the reactions for each part given in the introduction) 2 Explain the difference between the following two phrases. a Report the mass of the copper compound you started with b Report the mass of copper you started with 3 if you did not completely decompose all of the KClo, in your mixture in Part C, how would this influence the percentage of KCIO, you calculated? Be specific and explain your reasoning. percent of Copper, in the original sample. Equations 4 and 5 can be used to determine the percent of copper in If you can find the mass of copper in the product, you can determine the maw of copper, and imately the your sample: Mass of Cu in sample 2 Percentage of Cu in sample Mans of product x Theoretical percentage of Cu in Cuo 100 [Eq. 4) Mass of Cu in sample X 100 Mass of reactant Eq. 5) copper(II) carbonate hydroxide compounds, you can identify which compound you reacted. Now by comparing the percent of copper in your sample to the theoretical values for the two possible Composition of a Mixture Mixtures are an important part of chemistry, but many compounds have the same physical features as other compounds and, once mixed together, it is impossible to tell them apart. Percent composition can help us determine Reaction 5 Kcio, und Kai, both of which are white powder. Your goal is to determine the percentage orkaho in the mixture When heared, KCIO, decomposes according to the following reaction 2 KCIO, () 2 KCI (8) + 30, (O) As you hear the mixture, the mass of the system will initially decrease and then eventually remain constant. The total decrease in mass is due entirely to the loss of o, gas from the decomposition of KCIO, Using the mass of your original mixture, the final most of your product after heating, and the decomponition through action shown in Reactions you can determine the percent KCIO, in the original mixture using Equations 6 Mass of produced - Intial mass of mixture - Final mass of mixture [Eq. 6) Moles of O, produced Mass of O, produced [Eq. 73 Molar mass of O, Mass of KCIO, decomposed x 2 molos KCIO x Molar mass of KCIO TEq. 8) 3 moles o Note that the value 2/3 is the relationship of the Kio, and o, in the balanced equation Mass of KCIO, Percentage of KCIO, in original mixture Mass of mixture X 100 [Eq. 9) mixture can be determined as shown in Fusion 2 Percentage of compound Mass of compound Total mass of mixture Similar to recent spoon of an element in a compend the percent composite of and in 2 [Eq. 2] an unknown compound, and determine the percent of KCIO, in a mixture Identification of a Product 100 In this laboracy you will use percent composition to identify the product of a chemical reaction, Identify pressure, etc.). The ability to identify the product(s) is of vital importance in industry and research. After all, if you Chemical reactions of produce many different products depending on the conditions of the reaction (temperature, the product formed. Most mictals, when heated in air, react with either oxygen to form a metal oxide or with nitrogen In Part B. You will oxidize a sample of magnesium (Mg) metal and use the percent composition to determine don't know what you are producing the reaction is not very useful to form a metal nitride. The two possible reactions are: Reaction 1 2 Mg(s) + O. (o)-> 2 MgO (5) Reaction 2 3 Mg(s) + N) (9) - Ma.N, (s) Equation 3 to calculate the percent magnesium in the product: comparing that to the expected values for each possible compound. Once you have identified the product, use You can identify which product is formed by calculating the percent of magnesium in your product and Percentage of Mg Mass of Mo X 100 Mass of product MgO or Mg, N, -to determine which reaction occurred. Then compare this value to a theoretical percent composition of magnesium in the two possible products- [Eq. 3] Identification of a Reactant It is rarely possible to know the identity of a chemical at a glance. Many chemicals look, feel, and smell similar. Chemicals also can have similar names but very different formulas and reactivities. In Part C, you will be given according to the following reactions: azurite, Cuz(CO3)2(OH)2. When heated above 200C, both forms decompose to produce CO2, H,O, and Cuo copper(II) carbonate hydroxide. This compound can exist in two different forms: malachite, Cu,CO(OH)2, and Reaction 3 Cu CO3(OH)2 (s) = 2 CuO (s) + CO2 (g) + H2O (g) Reaction 4 Cus(CO)(OH)2 (5) 3 CuO (s) + 2 CO2 (g) + H2O (9) You will find the percent of copper in your sample and use that to determine which variety of the compound This means that the mass of copper in the final product is equal to the mass of copper in the original sample. fou have. Notice in the above reactions that all of the copper in the original compound becomes copper(II) oxide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


