Question: Part d and e please t Reactor selection and operating conditions. For each of the following sets of reactions, describe your reactor system and conditions
Part d and e please

t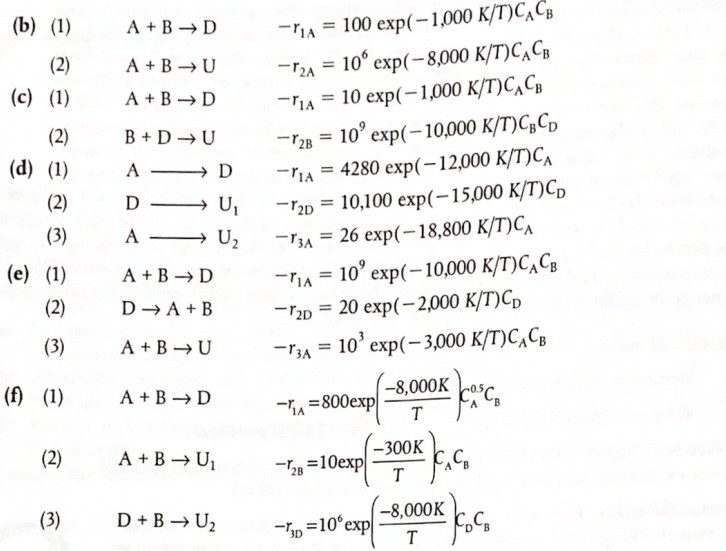
Reactor selection and operating conditions. For each of the following sets of reactions, describe your reactor system and conditions to maximize the selectivity to D. Make sketches where necessary to support your choices. The rates are in (mol/dm.s), and concentrations are in (mol/dm3). (a) (1) A + B +D -ra = 10 exp(-8,000 K/T)CACB (2) A + B U -r2A = 100 exp(-1,000 K/T)C? C312 1/2 (b) (1) A + B D = A + B U (2) (c) (1) A + B D B+D U A - D (2) (d) (1) (2) (3) - 14 = 100 exp(-1,000 K/T)CACB -2A = 10 exp(-8,000 K/T)CACB -TA - A = 10 exp(-1,000 K/T)CCB --2B 10' exp(-10,000 K/T)CC) -ra = 4280 exp(-12,000 K/T)CA -P20 = 10,100 exp(-15,000 K/T)CD -P3A = 26 exp(-18,800 K/T)CA exp(-10,000 K/T)CCB - rad = 20 exp(-2,000 K/T)Cp exp(-3,000 K/T), CB D- U A - U2 A + B D (e) (1) (2) A = 10 D A+B (3) A + B U P3A = 10 (f) (1) A+B D (-8,000K -1A =800expl coco T -300K -128=10exp T (2) A + B U kk (3) D+B U2 -8,000K -130 =10exp 1.C T
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


