Question: part D is the most important answer needed Problem 1. Use the phase diagram shown in the phase diagram below to determine the following. a)


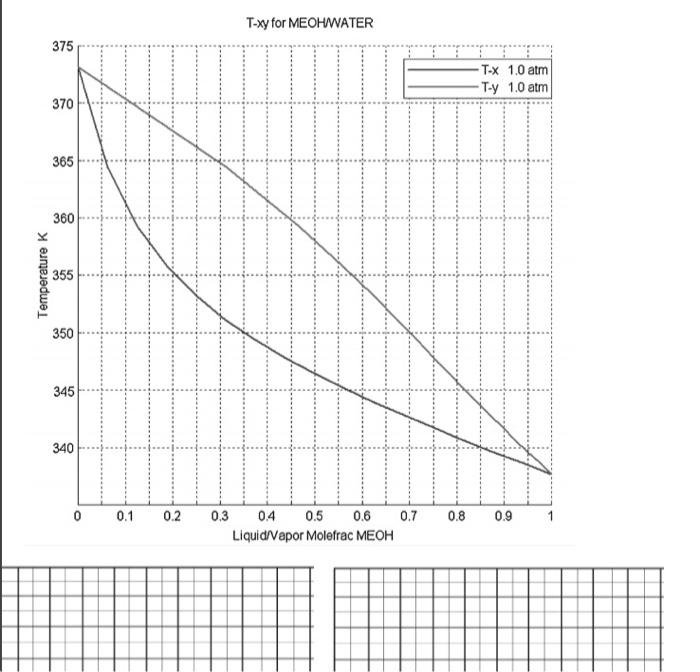
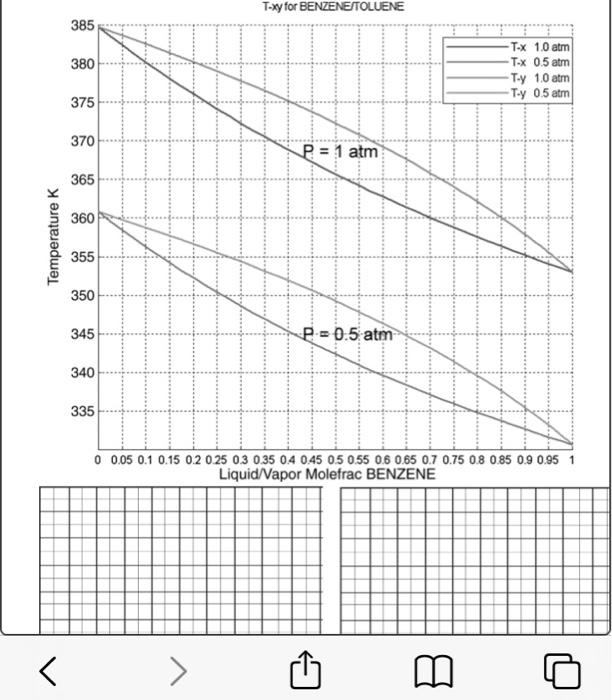
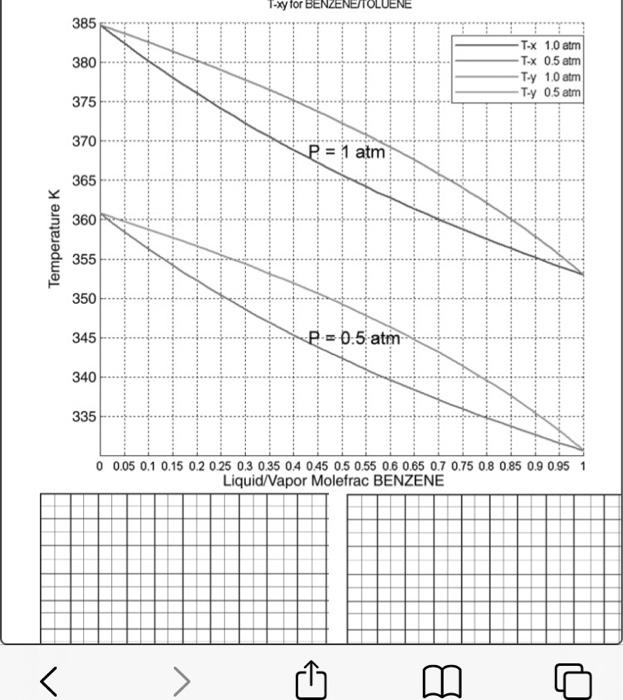
Problem 1. Use the phase diagram shown in the phase diagram below to determine the following. a) Does the methanol & water binary system plotted below obey Raoult's Law? How can we check? b) If not, how can we relate the equilibrium vapor and liquid phase compositions? c) What does the plot of y vs. x look like for this system? Construct a y-x diagram using the data shown in the T-xy diagram. d) What does the plot of g vs. x look like? Construct a g-x diagram using the data shown in the T-xy diagram. c) A flash separator operating at 101 kPa is fed a mixture that is one- third light key, two-thirds heavy key. i. Determine the temperature at which to operate the flash unit to vaporize one quarter of the feed. ii. Determine the liquid and vapor stream compositions achieved at this operating temperature. T-xy for BENZENETOLU 385 380 T-X 1.0 atm T-x 0.5 atm Ty 1.0 atm Tuy 0.5 atran 375 370 P = 1 atm 365 360 Temperature 355 350 345 P = 0.5 atm 340 335 0 0.05 0.1 0.15 0.2 0.25 0.3 0.35 0.4 0.45 0.5 0.55 0.6 0.65 0.7 0.75 0.8 0.85 0.9 0.95 1 Liquid/Vapor Molefrac BENZENE > B o
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


