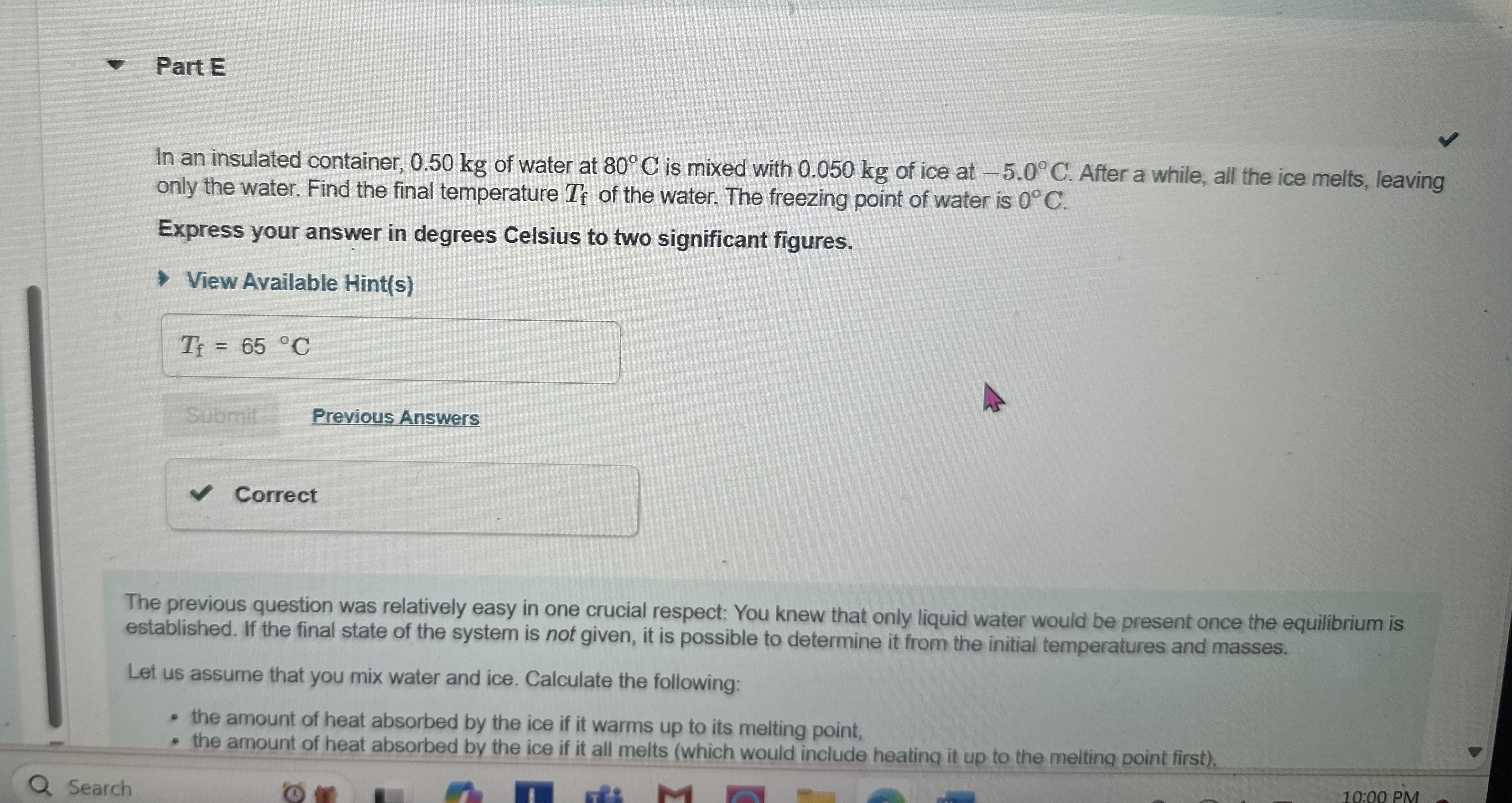
Question: Part E In an insulated container, 0 . 5 0 kg of water at 8 0 C is mixed with 0 . 0 5 0
Part E
In an insulated container, kg of water at is mixed with kg of ice at After a while, all the ice melts, leaving only the water. Find the final temperature of the water. The freezing point of water is
Express your answer in degrees Celsius to two significant figures.
View Available Hints
Previous Answers
The previous question was relatively easy in one crucial respect: You knew that only liquid water would be present once the equilibrium is established. If the final state of the system is not given, it is possible to determine it from the initial temperatures and masses.
Let us assume that you mix water and ice. Calculate the following:
the amount of heat absorbed by the ice if it warms up to its melting point,
the amount of heat absorbed by the ice if it all melts which would include heating it up to the melting point first CAN YOU PLEASE TELL ME WHY THIS answer is correct, sne why its not Thank you :
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


