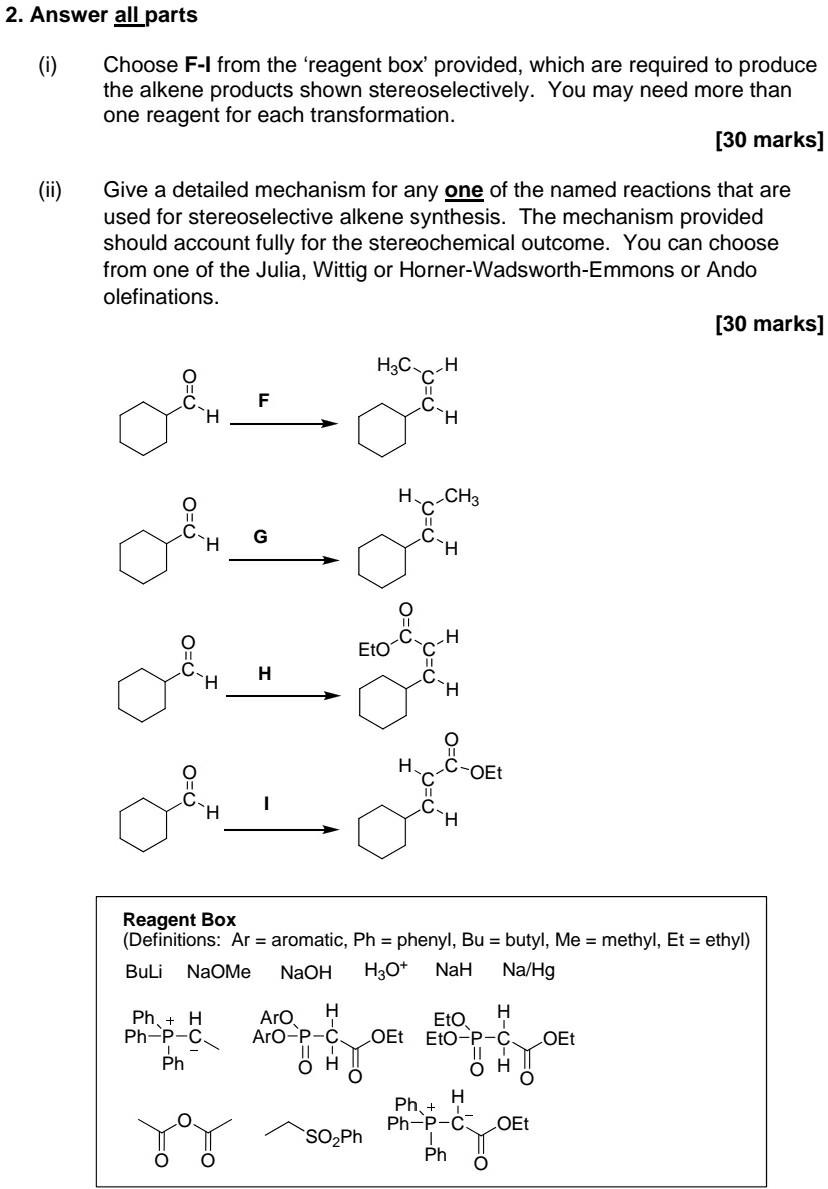
Question: Part i) & ii) Part iii) & iv) 2. Answer all parts Choose F-I from the 'reagent box' provided, which are required to produce the
Part i) & ii)
Part iii) & iv)
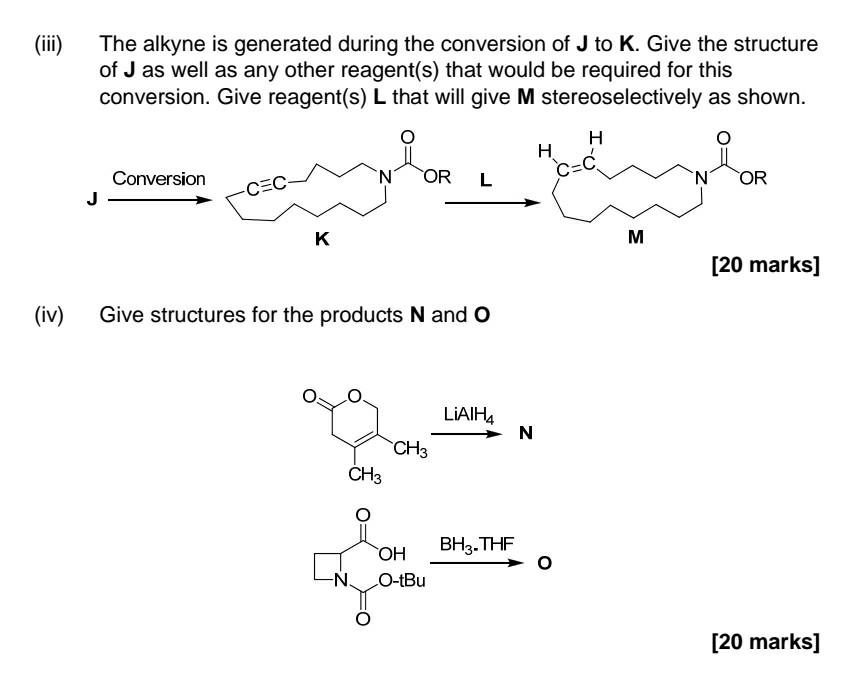
2. Answer all parts Choose F-I from the 'reagent box' provided, which are required to produce the alkene products shown stereoselectively. You may need more than one reagent for each transformation. [30 marks] Give a detailed mechanism for any one of the named reactions that are used for stereoselective alkene synthesis. The mechanism provided should account fully for the stereochemical outcome. You can choose from one of the Julia, Wittig or Horner-Wadsworth-Emmons or Ando olefinations. [30 marks] .. H Il F H H CH3 II H dhe OLIO Oh H C-OEt 11 H Reagent Box (Definitions: Ar = aromatic, Ph = phenyl, Bu = butyl, Me = methyl, Et = ethyl) Buli NaOME NaOH H30+ NaH Na/Hg Ph + H Ph-P-c. Ph Aro H Eto H Aro-p-c OEt Eto-p-C OEt II H O 0 H Ph-P-C OEt SO Ph Ph O Ph. + (iii) The alkyne is generated during the conversion of J to K. Give the structure of J as well as any other reagent(s) that would be required for this conversion. Give reagent(s) L that will give M stereoselectively as shown. H . Conversion N OR c=c L N OR CEC K M [20 marks] (iv) Give structures for the products N and O N O LIAIH4 N CH3 CH3 BH3. THE OH O-tBu N. [20 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


