Question: Part One Introduction to Basic Laboratory Techniques of EXPERIMENT 1 Solubility Solubility Polarity Acid-base churmistry Critical thinking application Having a good comprehension of solubility behavior


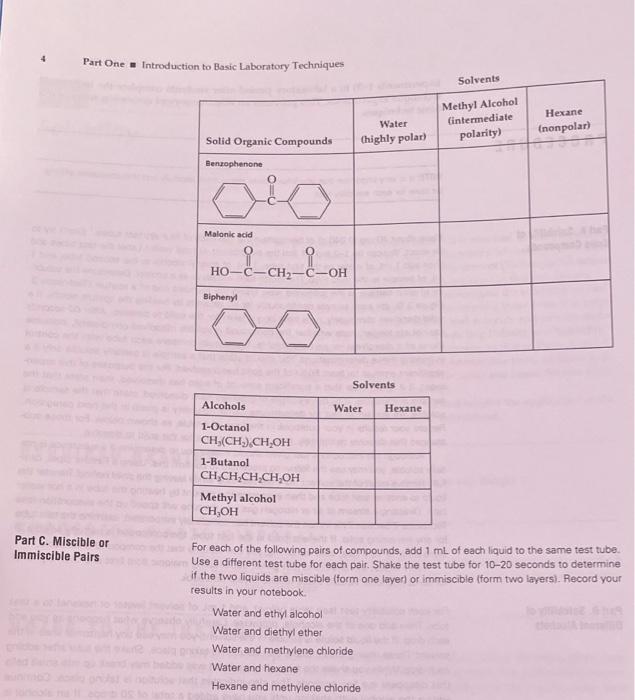

Part One Introduction to Basic Laboratory Techniques of EXPERIMENT 1 Solubility Solubility Polarity Acid-base churmistry Critical thinking application Having a good comprehension of solubility behavior is essential for understanding many procedures and techniques in the organic Chemistry laboratory. For a thor- ough discussion of solubility, read the chapter on this concept (Technique 10) before proceeding as an understanding of this material is assumed in this experiment. In Parts A and B of this experiment, you will investigate the solubility of vari ous substances in different solvents. As you are performing these tests, it is help- ful to pay attention to the polarities of the solutes and solvents and to even make predictions based on them (see "Guidelines for Predicting Polarity and Solubility" Technique 10, Section 10:4). The goal of Part Cis similar to that of Parts A and B, except that you will be looking at miscible and immiscible pairs of liquids. In Part D, you will investigate the solubility of organic acids and bases. Section 10:28 will help you understand and explain these results In Part E. you will perform several cercises that involve the application of the solubility principles learned in Parts A-Dof this experiment REQUIRED READING Neter Technique 5 Technique 10 Measurement of Volume and Weight Solubility SUGGESTED WASTE DISPOSAL Dispose of all wastes containing methylene chloride into the container marked for halogenated waste Place all other organic wastes into the nonhalogenated organic waste container. NOTES TO THE INSTRUCTORS In Part A of the procedure, it is important that students follow the instructions care fully. Otherwise, the results may be difficult to interpret. It is particularly impor- tant that consistent stirring is done for each solubility test. This can be done most easily by using the spatula found in your drawer, We have found that some students have difficulty performing Critical- Thinking Application 2 in Part on the same day that they complete the rest of this experiment. Many students need time to assimilate the material in the experi- ment before they can complete this exercise successfully. One approach is to assign Critical Thinking Applications from several technique experiments (for example, Experiment1 Solubility Experiments 1-3) to a laboratory period after students complete the individual technique experiments. This provides an effective way of reviewing some of the basic techniques PROCEDURE NOTE: It is very important that you follow these instructions carefully and that consistenting be done for each solubility test Part A. Solubility of Solid Compounds Place about 40 mg 10040 gof benzophenone into each of four dry test tubes' (Don't try to be exact. You can be 1-2 mg oft and the experiment will still work Label the fest tubes and then add 1 ml of water to the first tubo, 1 ml of methyl alcohol to the second tube, and 1 mL of hexane to the third tube The fourth tube will serve as a control Determine the solubl. ity of each samole in the following way. Using the rounded end of a spatula Technique 3. Figure 3.5, str each sample continuously for 60 seconds by twirling the spatula rapidly. If solid dissolves completely note how long it takes for the sold to dissolve. After 60 seconds Ido not stir longerl, note whether the compound is soluble (dissolves completely insoluble Inone of it dissolves or partially soluble. You should compare each tube with the control in making these determinations. You should state that a sample is partially soluble only if a significant amount at least 50% of the solid has dissolved. For the purposes of this experie ment, if it is not clear that a significant amount of solid has dissolved, then state that the samole is insolubleIf but a couple of granules have dissolved, state that the sample is soluble. An additional hint for determining partial solubility is given in the next paragraph Record your results in your notebook in the form of a table, as shown below. For those sub- stances that dissolve completely note how long it took for the sold to dissolve Although the instructions just given should enable you to determine if a substance is par taly soluble, you may use the following procedure to confirm this. Using a Pastouppet care fully remove most of the solvent from the test tube while leaving the solid befind. Transfer the liquid to another test tube and then evaporate the solvent by heating the tube in a hot water bath Directing a stream of dit or nitrogen gas into the tube will speed up the evaporation so Technique Section 2101. When the solvent has completely evaporated, camine the test tube for any remaining sold. If there is sold in the test tube, the compound is partially soluble. If there is no or very little, sold remaining, you can assume that the compound is insoluble Now repeat the directions just given substituting malonic acid first and then biphenyl for benzophenone Record these results in your notebook Part B. Solubility of Different Alcohols For each solubility test (see table belowi, add 1 mL of solvent Iwater or hexanel to a fost tube. Then add one of the alcohols, dropwise. Carefully observe what happens as you add each drop. If the liquid solute is soluble in the solvent, you may see tiny horizontal lines in the solvent. These mixing lines indicate that solution taking place Shake the tube after adding each drop. While you shake the tube, the liquid that was added may break up into small bals that disappear in a few seconds. This also indicates that solution is taking place. Continue adding the alcohol with shaking until you have added a total of 20 drops. If an alcohol is partially soluble, you wil observe that at first the drops will dissolve, but eventually a second layer of liquidfundissolved alcohol will form in the test tubo Record your results isoluble insoluble, or partially soluble in your notebook in table form Note to be betructor: Grind up the benzopherone flakes into a powder Part One Introduction to Basic Laboratory Techniques Solvents Water Chighly polar) Methyl Alcohol intermediate polarity) Hexane (nonpolar) Solid Organic Compounds Benzophenone Malonic acid 8-CHI HO-C-CH2-C-OH Biphenyl Solvents Water Hexane Alcohols 1-Octanol CH,(CH).CH OH 1-Butanol CH,CH,CH.CH,OH Methyl alcohol CH,OH Part C. Miscible or Immiscible Pairs For each of the following pairs of compounds, add 1 ml of each liquid to the same test tube. Use a different test tube for each pair. Shake the test tube for 10-20 seconds to determine if the two liquids are miscible (form one layer or immiscible (form two layers). Record your results in your notebook Water and ethyl alcohol Water and diethyl ether Water and methylene chloride Water and hexane Hexane and methylene chloride C. Balanced chemical equation and Theoretical Yield (if applicable, must be handwritten, never typed). Present the balanced chemical equation and calculate the theoretical yield for all reactions, showing the appropriate math. D. Reagent Table (Must be handwritten, never typed): List all chemicals to be used, including solvents. List any hazards associated with each chemical. When a reaction is run, give the quantity of each reagent in grams or mL, along with MW, density and moles. E. Mechanism (if applicable, must be handwritten, never typed): Write clear stepwise mechanisms for all synthetic transformations, showing important intermediates where appropriate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


