Question: Part-2 (15 points) Which of these elements would you expect to form the following with nickel: (a) A substitutional solid solution having complete solubility
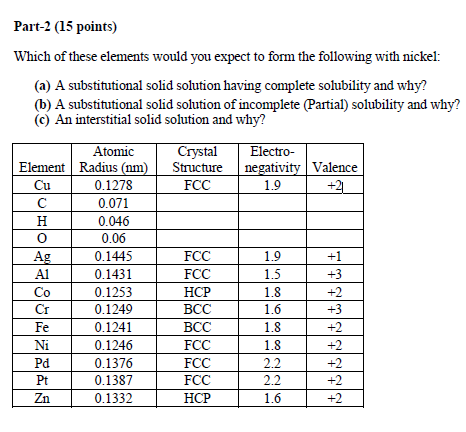
Part-2 (15 points) Which of these elements would you expect to form the following with nickel: (a) A substitutional solid solution having complete solubility and why? (b) A substitutional solid solution of incomplete (Partial) solubility and why? (c) An interstitial solid solution and why? Element Cu HO Ag A1 Co Fe Ni Pd Pt Zn Atomic Radius (nm) 0.1278 0.071 0.046 0.06 0.1445 0.1431 0.1253 0.1249 0.1241 0.1246 0.1376 0.1387 0.1332 Crystal Structure FCC FCC FCC HCP BCC BCC FCC FCC FCC HCP Electro- negativity Valence 1.9 +21 1.9 1.5 1.8 1.6 1.8 1.8 2.2 2.2 1.6 +1 +3 +2 +3 +2 +2 +2 +2 +2
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


