Question: PartB & C please 1302 - 4.2 - Exercise 18.72 - Copy What is the initial cell potential? Express your answer using two decimal places.
PartB & C please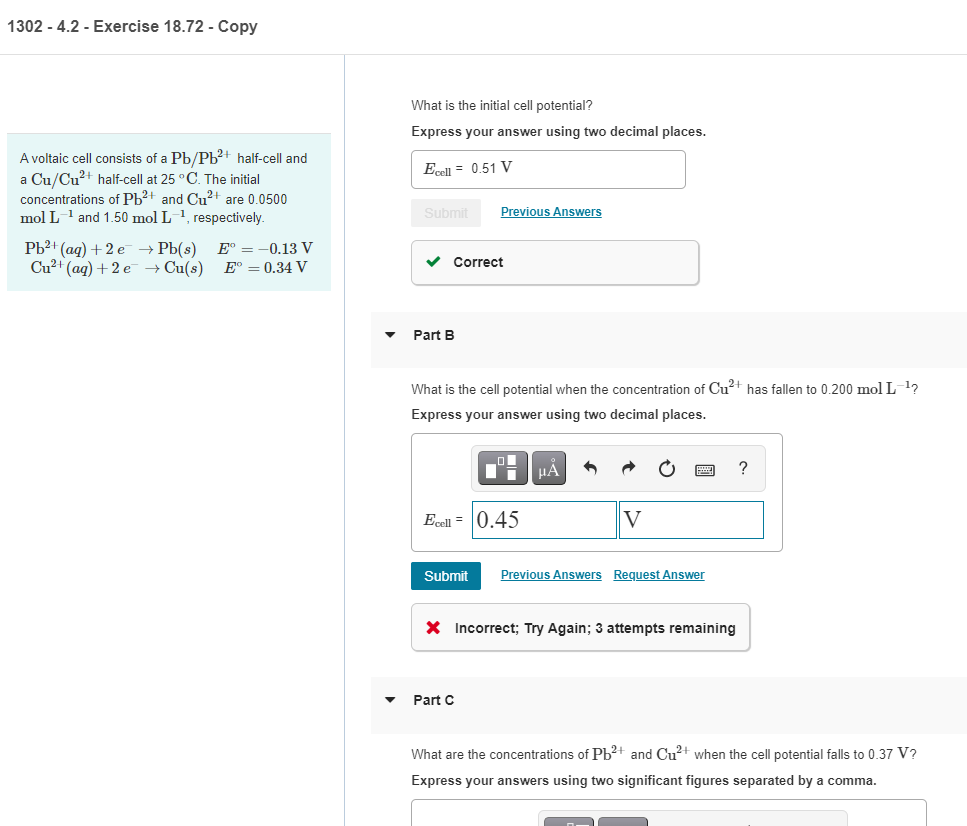
1302 - 4.2 - Exercise 18.72 - Copy What is the initial cell potential? Express your answer using two decimal places. Ecol = 0.51 V A voltaic cell consists of a Pb/Pb2+ half-cell and a Cu/Cu+ half-cell at 25C. The initial concentrations of Pb2+ and Cu2+ are 0.0500 mol L-1 and 1.50 mol L -1, respectively Pb2+ (aq) +2 e Pb(s) E = -0.13 V Cu(aq) +2e + Cu(s) E = 0.34 V Submit Previous Answers Correct Part B What is the cell potential when the concentration of Cu+ has fallen to 0.200 mol L-1? Express your answer using two decimal places. ? Ecell = 0.45 V Submit Previous Answers Request Answer X Incorrect; Try Again; 3 attempts remaining Part C What are the concentrations of Pb2+ and Cu+ when the cell potential falls to 0.37 V? Express your answers using two significant figures separated by a comma
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


