Question: Particle #2 - Formaldehyde molecule ( CH2O) Question 26 - 30 This molecule has three bonds: two CH and one CO bonds. Answer the following
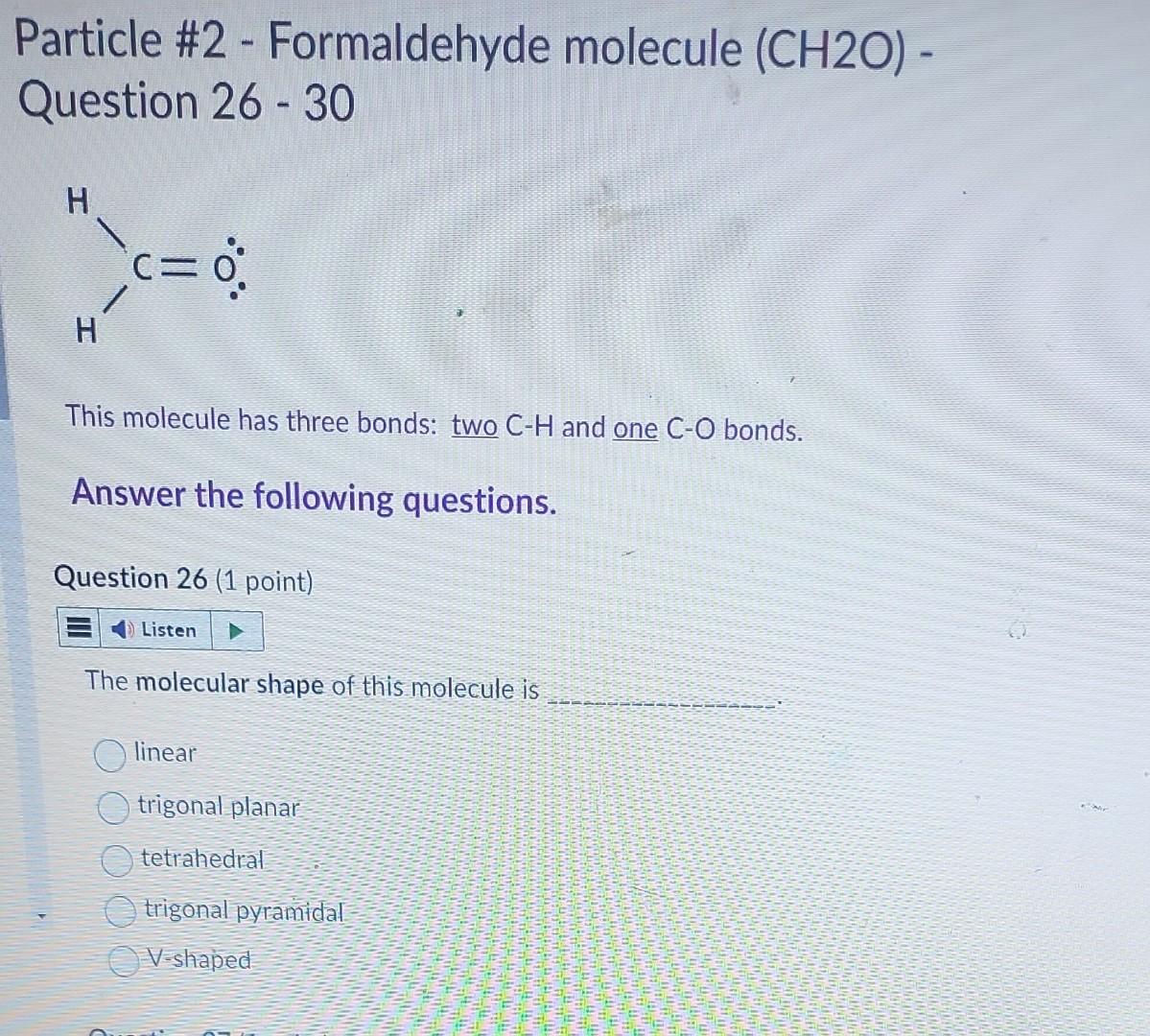
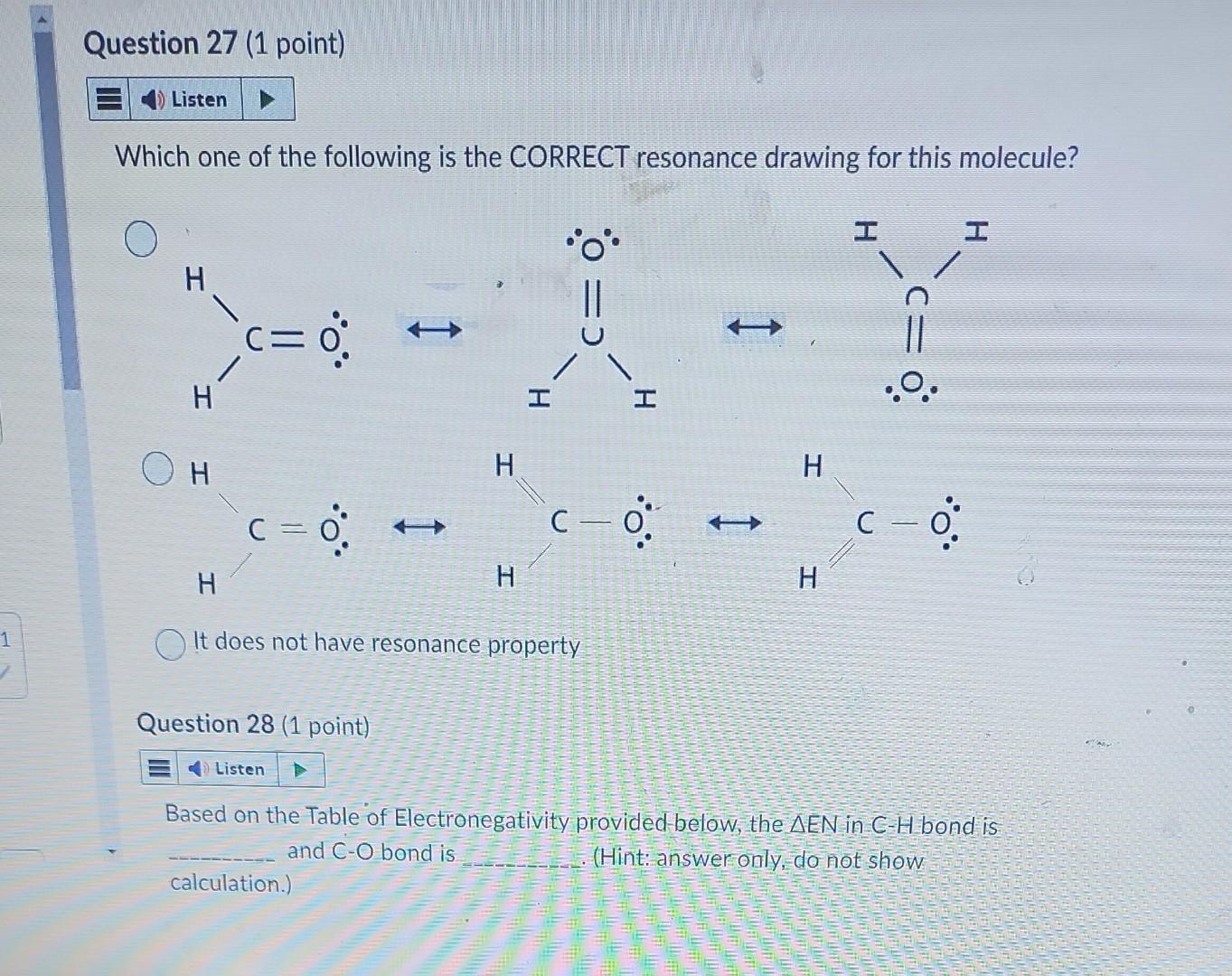
Particle \#2 - Formaldehyde molecule ( CH2O) Question 26 - 30 This molecule has three bonds: two CH and one CO bonds. Answer the following questions. Question 26 (1 point) The molecular shape of this molecule is linear trigonal planar tetrahedral trigonal pyramidal V-shaped Which one of the following is the CORRECT resonance drawing for this molecule? It does not have resonance property Question 28 (1 point) Based on the Table of Electronegativity provided-below, the EN in CH bond is and CO bond is (Hint: answer only, do not show calculation.) Both CH and CO bonds are polar bonds with different EN. Therefore, the overall electron charge distribution of molecule is symmetrical (i.e. bond dipole moments in molecule are cancelled) unsymmetrical (i.e. bond dipole moments in molecule are NOT cancelled.) Question 30 (1 point) This molecule has polar bond and is polar molecule polar bond but is nonpolar molecule nonpolar bond and is nonpolar molecule
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


