Question: Pease answer a&b with work shown. The phosphate buffer solution has a ph=7.5, pka=2.13, and (A-/HA) ratio= 2.4x10^5 1. This problem will be done for
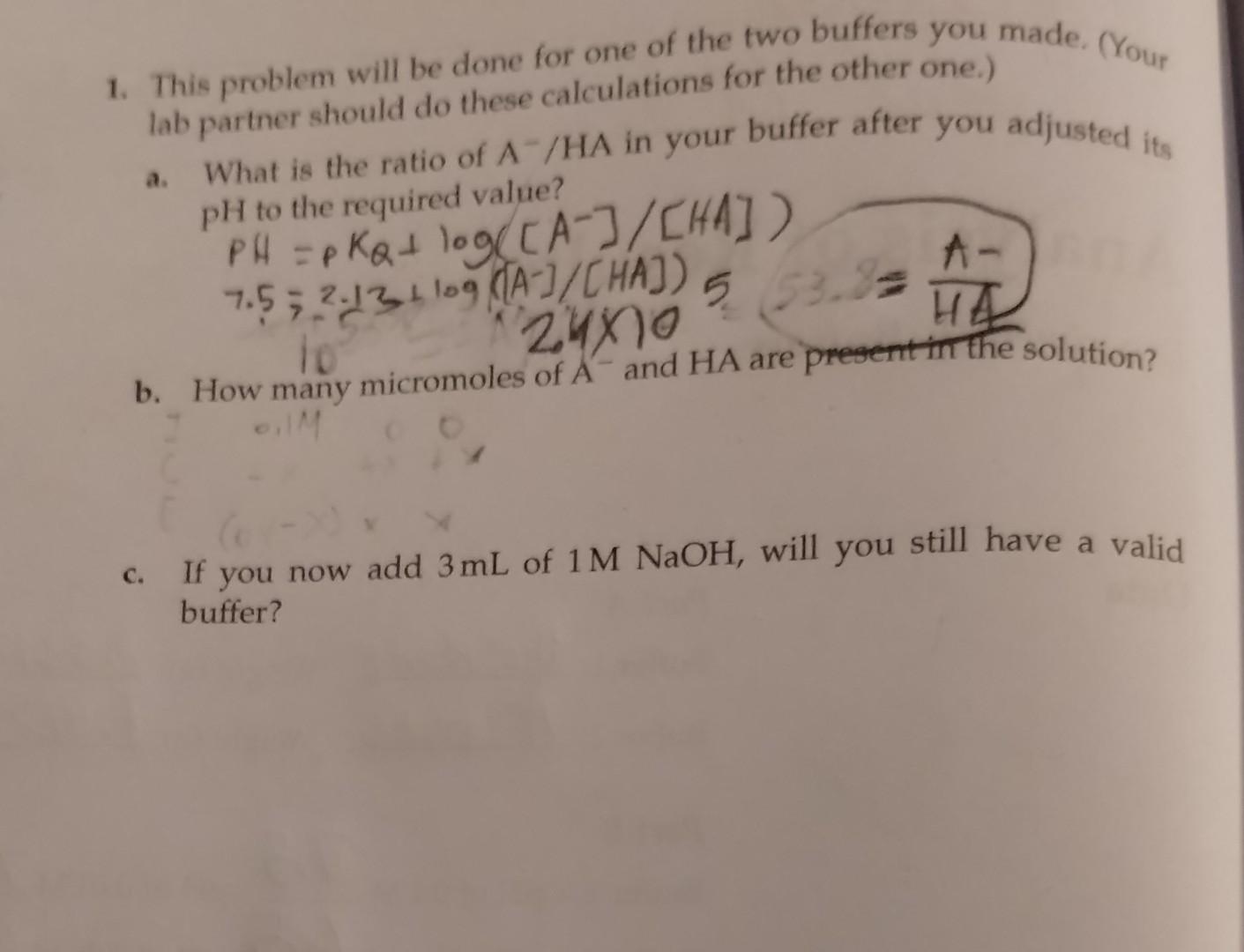
Pease answer a&b with work shown. The phosphate buffer solution has a ph=7.5, pka=2.13, and (A-/HA) ratio= 2.4x10^5
1. This problem will be done for one of the two buffers you made. (Your lab partner should do these calculations for the other one.) a. What is the ratio of A/HA in your buffer after you adjusted its PH to the required value? Pf=PKQ+log([A]/[HA]) 7.52.13+log([A]/[HA])5 b. How many micromoles of Aand HA are present in the solution? c. If you now add 3mL of 1MNaOH, will you still have a valid buffer? 1. This problem will be done for one of the two buffers you made. (Your lab partner should do these calculations for the other one.) a. What is the ratio of A/HA in your buffer after you adjusted its PH to the required value? Pf=PKQ+log([A]/[HA]) 7.52.13+log([A]/[HA])5 b. How many micromoles of Aand HA are present in the solution? c. If you now add 3mL of 1MNaOH, will you still have a valid buffer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


