Question: percent composition by mass is as follows. A solution that is prepared by dissolving 3.150 grams of the substance in 25.00 grams of benzene, C6H6,
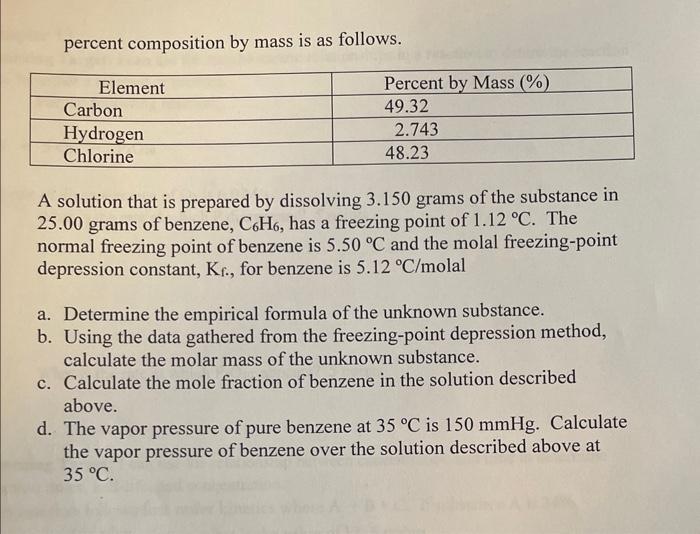
percent composition by mass is as follows. A solution that is prepared by dissolving 3.150 grams of the substance in 25.00 grams of benzene, C6H6, has a freezing point of 1.12C. The normal freezing point of benzene is 5.50C and the molal freezing-point depression constant, Kf, for benzene is 5.12C/ molal a. Determine the empirical formula of the unknown substance. b. Using the data gathered from the freezing-point depression method, calculate the molar mass of the unknown substance. c. Calculate the mole fraction of benzene in the solution described above. d. The vapor pressure of pure benzene at 35C is 150mmHg. Calculate the vapor pressure of benzene over the solution described above at 35C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


