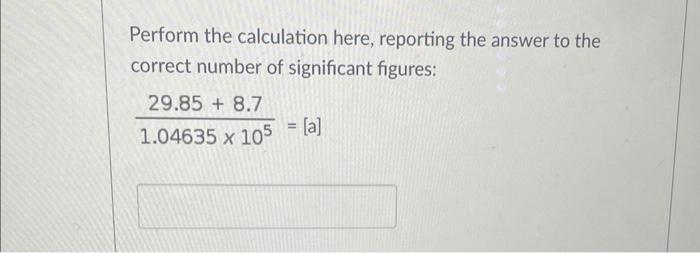
Question: Perform the calculation here, reporting the answer to the correct number of significant figures: 1.0463510529.85+8.7=[a] Give the correct answer to the calculation: 45.850.293+105.679 When 36.5g
![of significant figures: 1.0463510529.85+8.7=[a] Give the correct answer to the calculation: 45.850.293+105.679](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f6fca89ec93_56866f6fca84ab48.jpg)
![When 36.5g of hydrochloric acid reacts with excess magnesium metal, [a] grams](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f6fca927b85_56866f6fca8ccd09.jpg)
Perform the calculation here, reporting the answer to the correct number of significant figures: 1.0463510529.85+8.7=[a] Give the correct answer to the calculation: 45.850.293+105.679 When 36.5g of hydrochloric acid reacts with excess magnesium metal, [a] grams of hydrogen gas are formed. 2HCl(aq)+Mg(s)MgCl2(aq)+H2(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


