Question: permutations A solution M 1 containing 1 8 % K C l , 3 % K 2 S O 4 and 7 9 % water
permutations
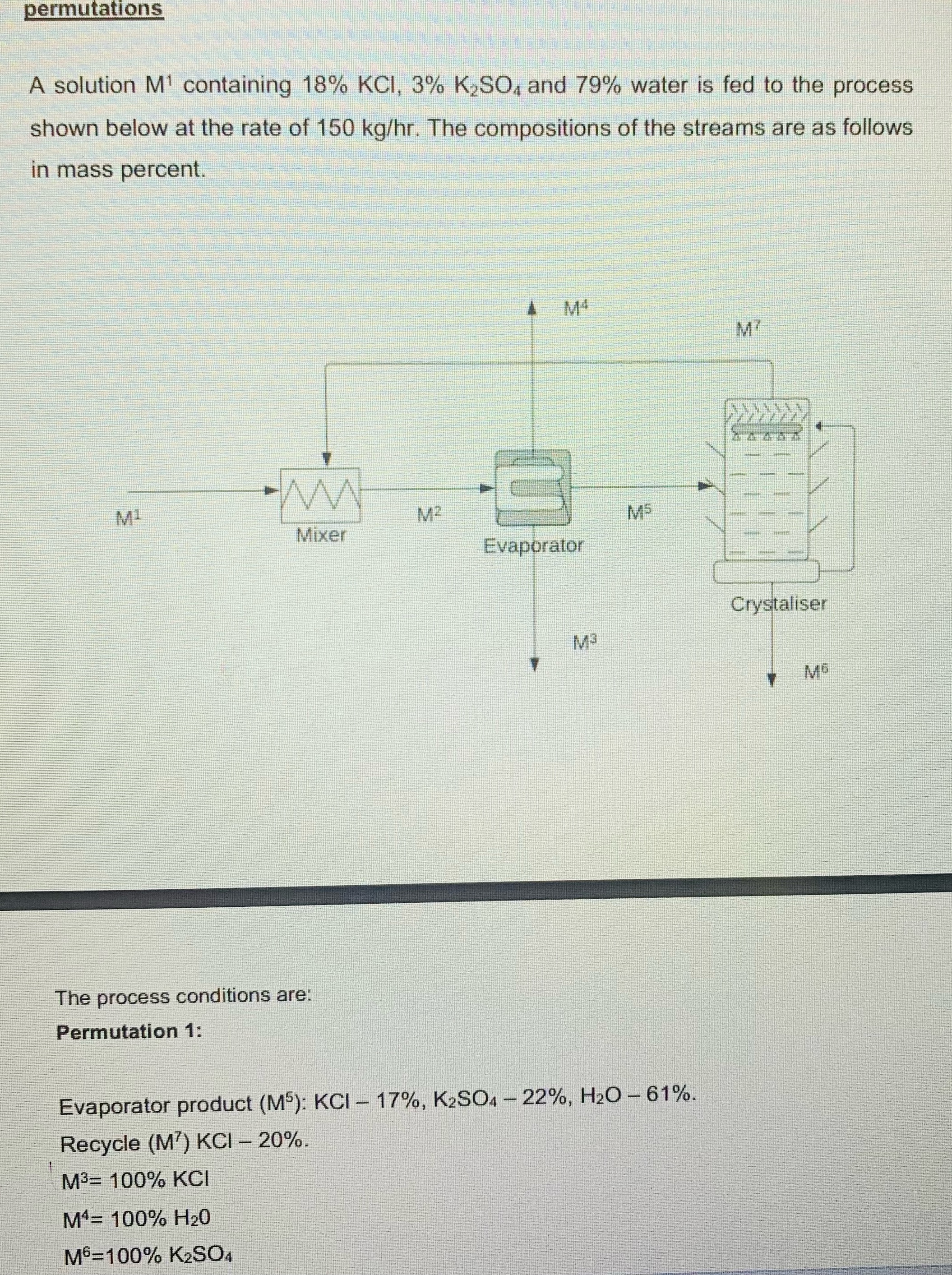
A solution containing and water is fed to the process shown below at the rate of The compositions of the streams are as follows in mass percent.
The process conditions are:
Permutation :
Evaporator product :
Recycle
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


