Question: From tubes 3-8, write hydrolysis equation (water added) to the substance (NaCl, ZnCl2, etc.) calculate concentration of H3O+ and OH- calculate ka and kb with
From tubes 3-8, write hydrolysis equation (water added) to the substance (NaCl, ZnCl2, etc.)
calculate concentration of H3O+ and OH-
calculate ka and kb with equation: kw=ka x kb
kw= 1.0 x 10^-4
pH for test tubes 1-8:
1: 4-6
2: 7
3: 4-6
4: 7
5: 4-6
6: 4-6
7: 7
8: 12-14
?
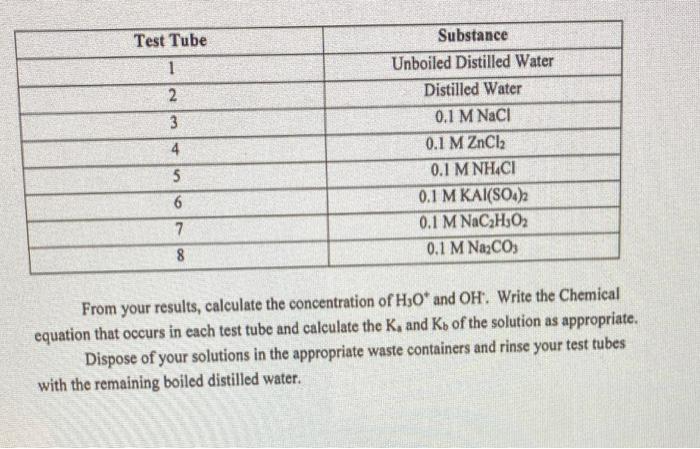
Test Tube 1 2 3 4 5 6 7 8 Substance Unboiled Distilled Water Distilled Water 0.1 M NaCl 0.1 M ZnCl 0.1 M NH.C1 0.1 M KAI(SO4)2 0.1 M NaCHO 0.1 M NaCO3 From your results, calculate the concentration of H3O* and OH. Write the Chemical equation that occurs in each test tube and calculate the K, and Kb of the solution as appropriate. Dispose of your solutions in the appropriate waste containers and rinse your test tubes with the remaining boiled distilled water.
Step by Step Solution
3.53 Rating (150 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
636470507edaf_239348.pdf
180 KBs PDF File
636470507edaf_239348.docx
120 KBs Word File


