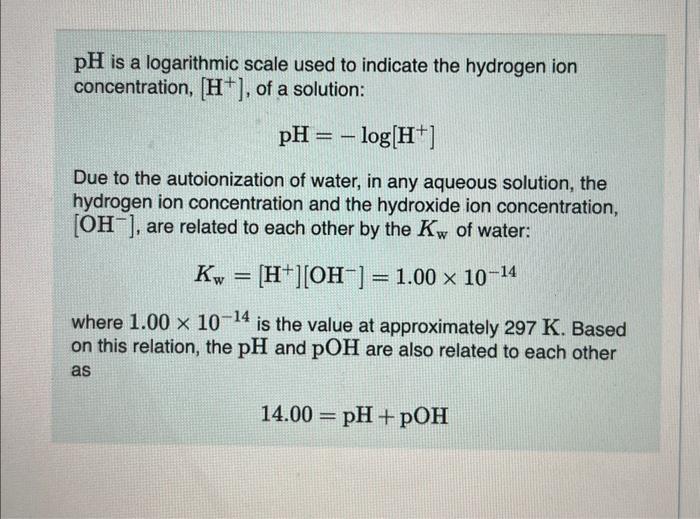
Question: pH is a logarithmic scale used to indicate the hydrogen ion concentration, [H+], of a solution: pH=log[H+] Due to the autoionization of water, in any
![concentration, [H+], of a solution: pH=log[H+] Due to the autoionization of water,](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8e46a06d64_43366f8e469a1364.jpg)
pH is a logarithmic scale used to indicate the hydrogen ion concentration, [H+], of a solution: pH=log[H+] Due to the autoionization of water, in any aqueous solution, the hydrogen ion concentration and the hydroxide ion concentration, [OH], are related to each other by the Kw of water: Kw=[H+][OH]=1.001014 where 1.001014 is the value at approximately 297K. Based on this relation, the pH and pOH are also related to each other as 14.00=pH+pOH 0.90g of sodium hydroxide (NaOH) pellets are dissoived in water to make 2.0L of solution. What is the pH of this solution? Express the pH numerically to two decimal places. View Available Hint(s) Correct Because the concentration had two significant figures, the pH has two digits atter the decimal point. What is the pOH of the solution in Part B? Express the pOH numerically to two decimal places
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


